Onset and Duration of imAEs
General Considerations
Onset and Duration of imAEs

imAEs may occur at any time during treatment. 1
- Most imAEs will occur within the first few weeks or months (median onset is usually 2–16 weeks) after
treatment initiation.2
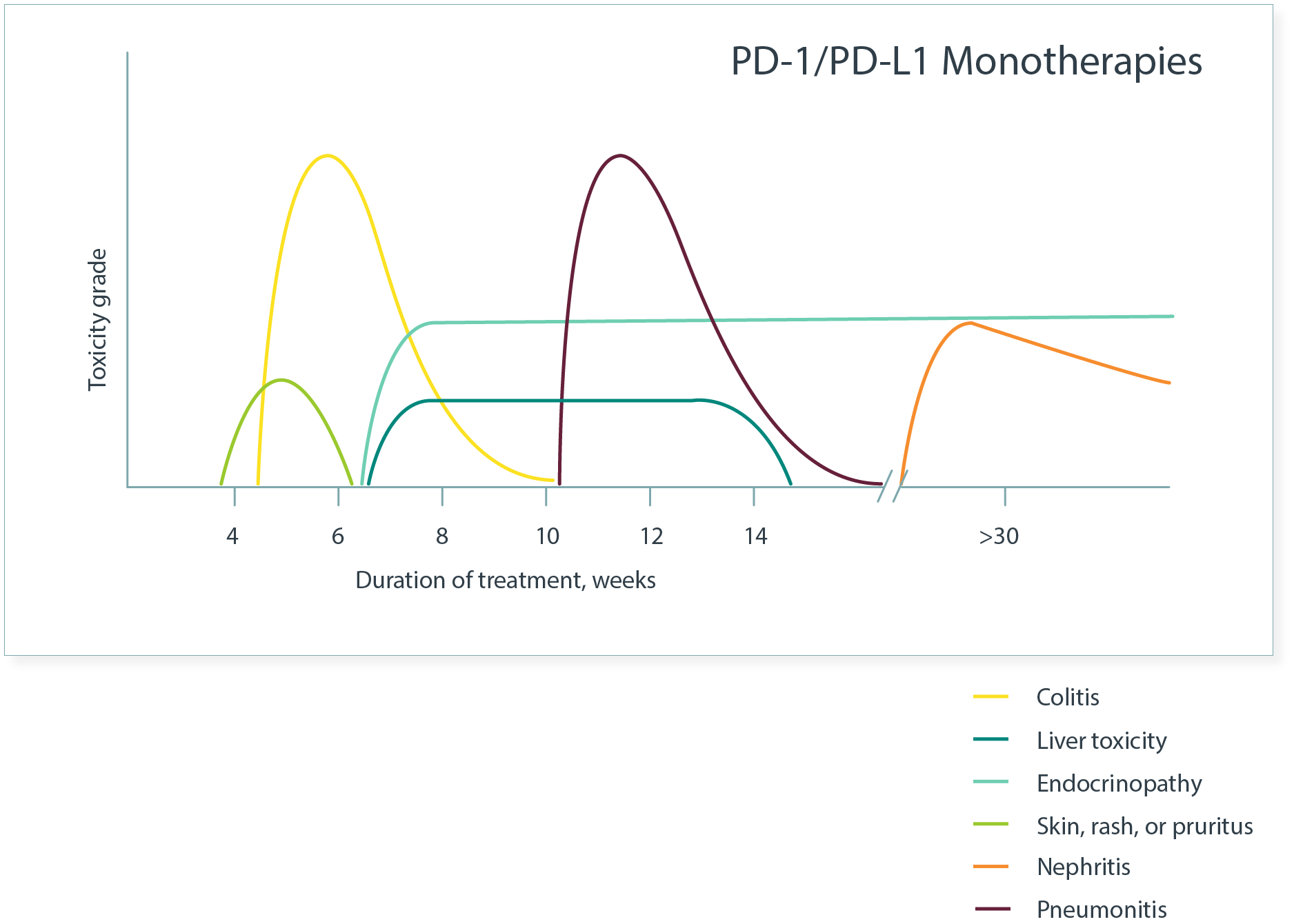
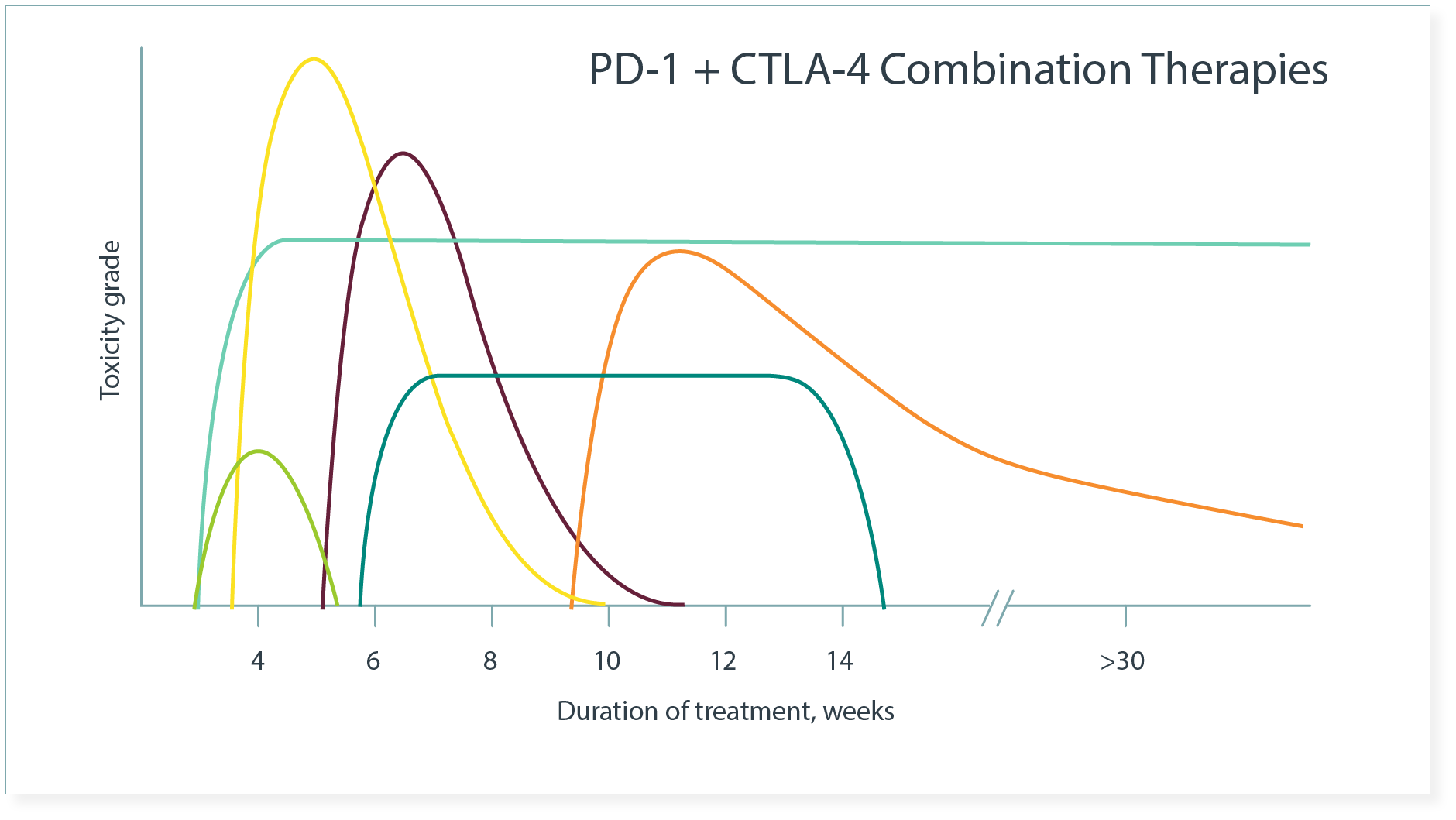
- Overall, imAEs in patients receiving combination ICIs appear to have an earlier onset than the same imAEs in those receiving monotherapies.3

The pattern of onset may vary by organ system.4
- Dermatologic imAEs commonly are the first to emerge.1

Occurrence of different imAEs can be simultaneous or can emerge one after another, and imAEs may persist beyond cessation of treatment.5
Kinetics of Common imAEs3
References:
- Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018 Jan 11;378(2):158-168.
- Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020 May7;6(1):38.
- Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol.2019 Sep;16(9):563-580.
- NCCN (National Comprehensive Cancer Network) V.1.2023. Accessed at:https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Last accessed:1-5-2023.
- Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021 Jun;9(6):e002435.
MSD Egypt, 67 – El Tesseen St., Address Building, Fifth Settlement, New Cairo.
In case you need any updates or you have an inquiry or need to report on an adverse reaction, you can contact:
Veeva Code: EG-KEY-00280
Expiration Date: 10/07/2024





