Infusion-related Reactions

OVERVIEW
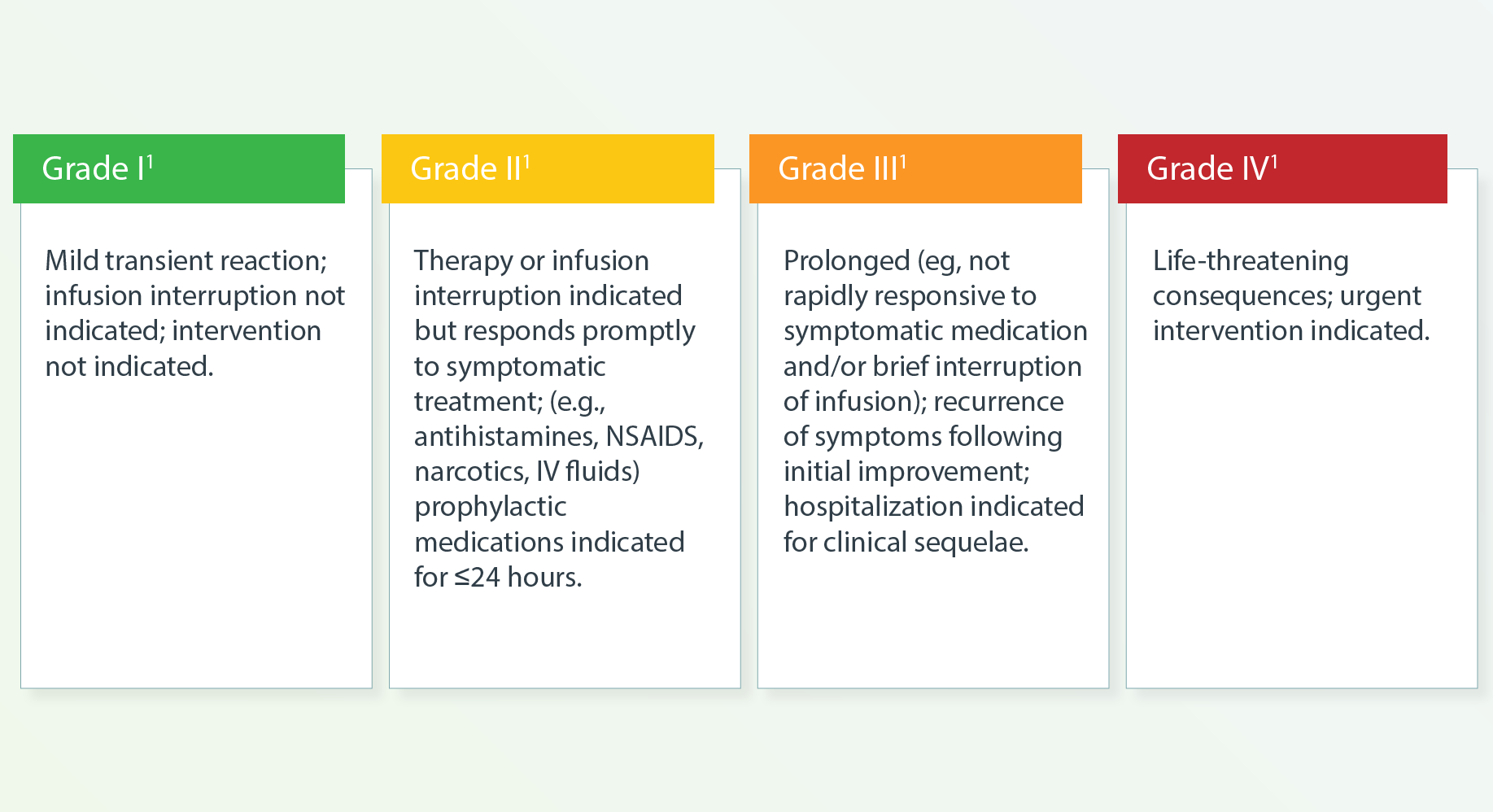
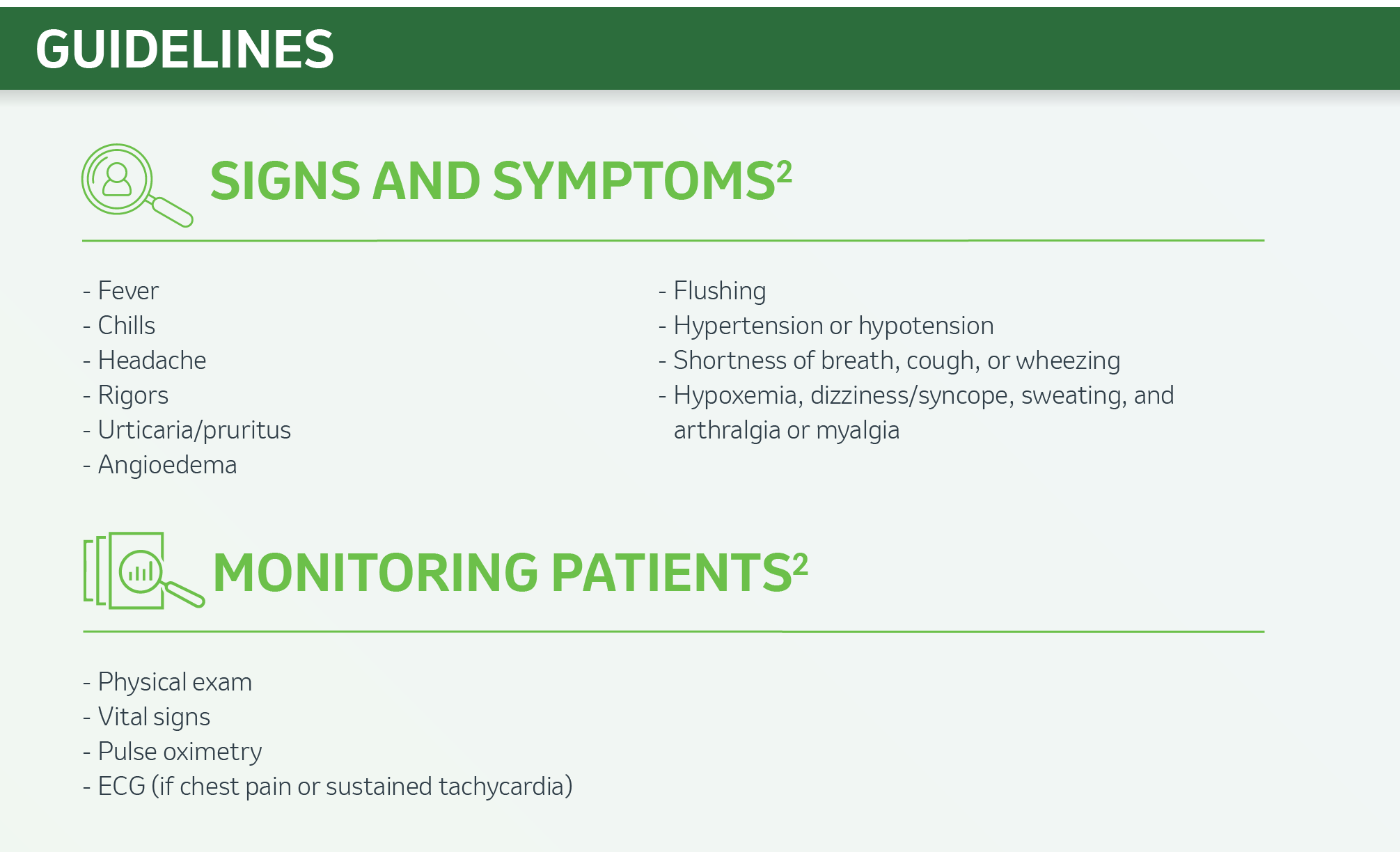
Infusion-related Reactions
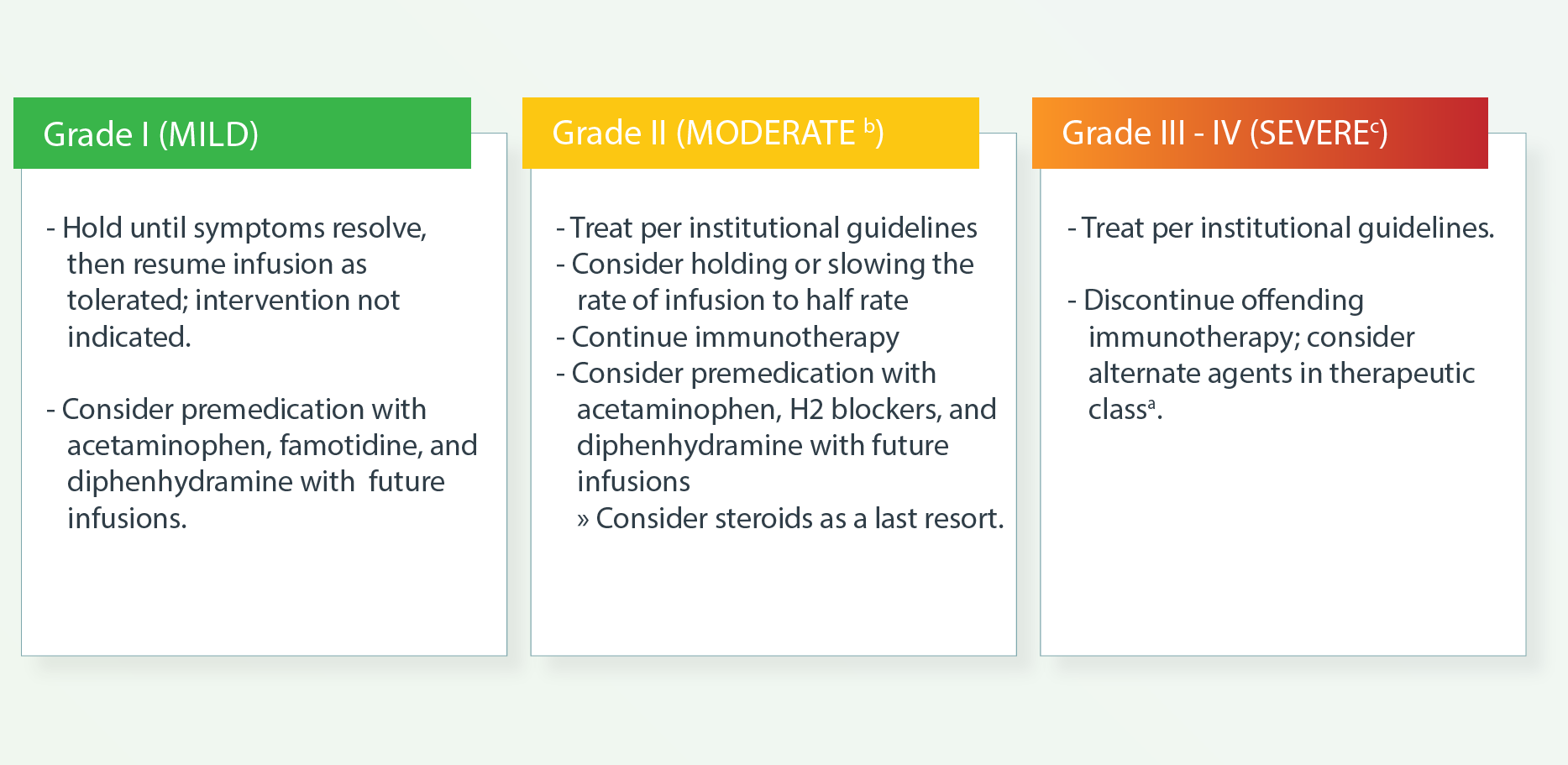
b Therapy or infusion interruption indicated but responds promptly to symptomatic treatment (eg, antihistamines, acetaminophen,
nonsteroidal anti-inflammatory drugs [NSAIDs], narcotics, intravenous [IV] fluids); prophylactic medications indicated for 24
hours.
c Prolonged (eg, not rapidly responsive to symptomatic medication and/or brief interruption of infusion); recurrence of symptoms following initial improvement. Hospitalization indicated; life-threatening consequences; urgent intervention.
d If infusion reactions that are resistant to standard therapy occur in patients receiving PD-L1 inhibitors, consider switching to a PD-1 inhibitor for subsequent treatments. There are no data to guide the use of alternate immune checkpoint inhibitors (ICIs)
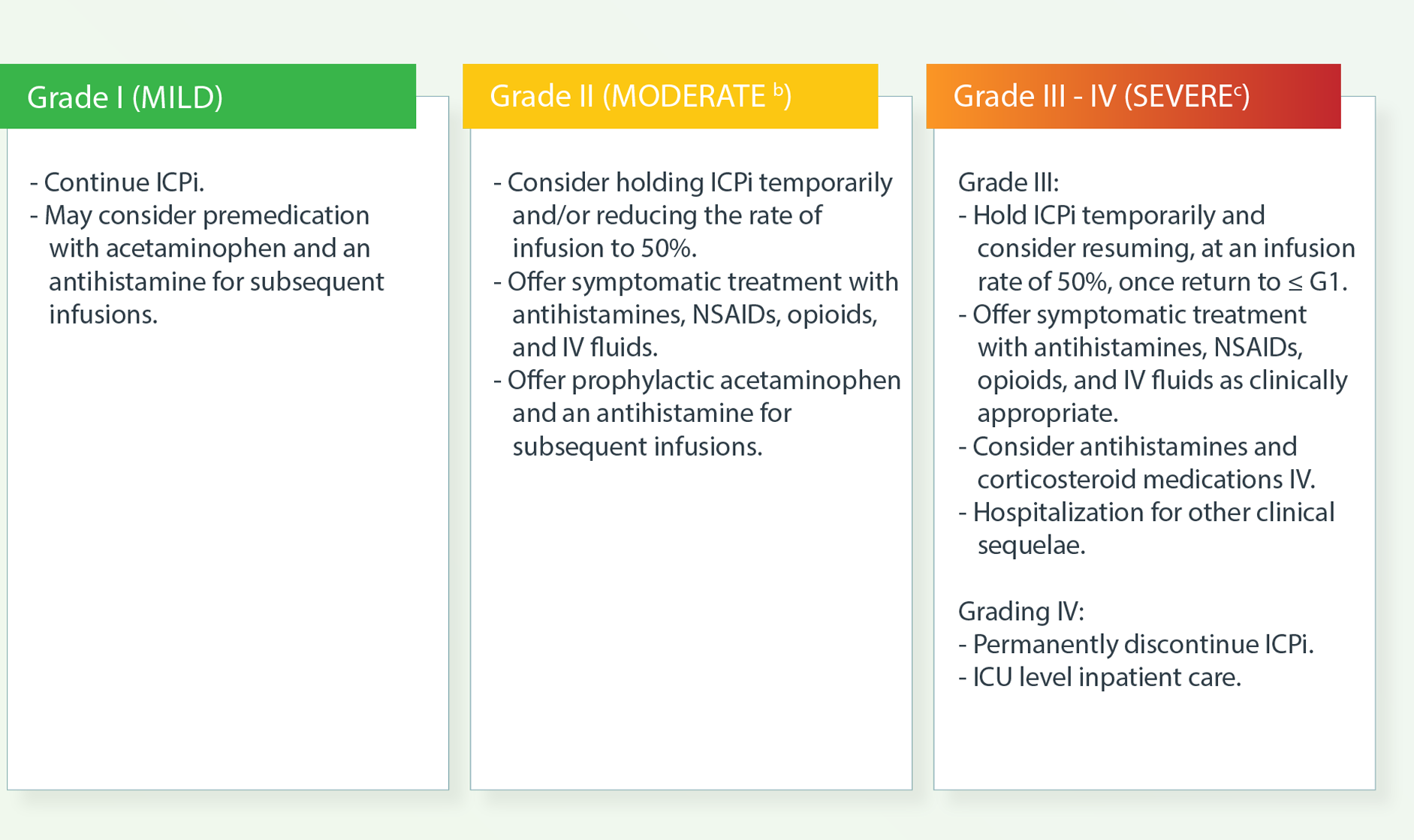
ICPi, immune checkpoint inhibitor; ICU, intensive care unit; IRR, infusion-related reaction; IV, intravenous; NSAID, nonsteroidal antiinflammatory drug
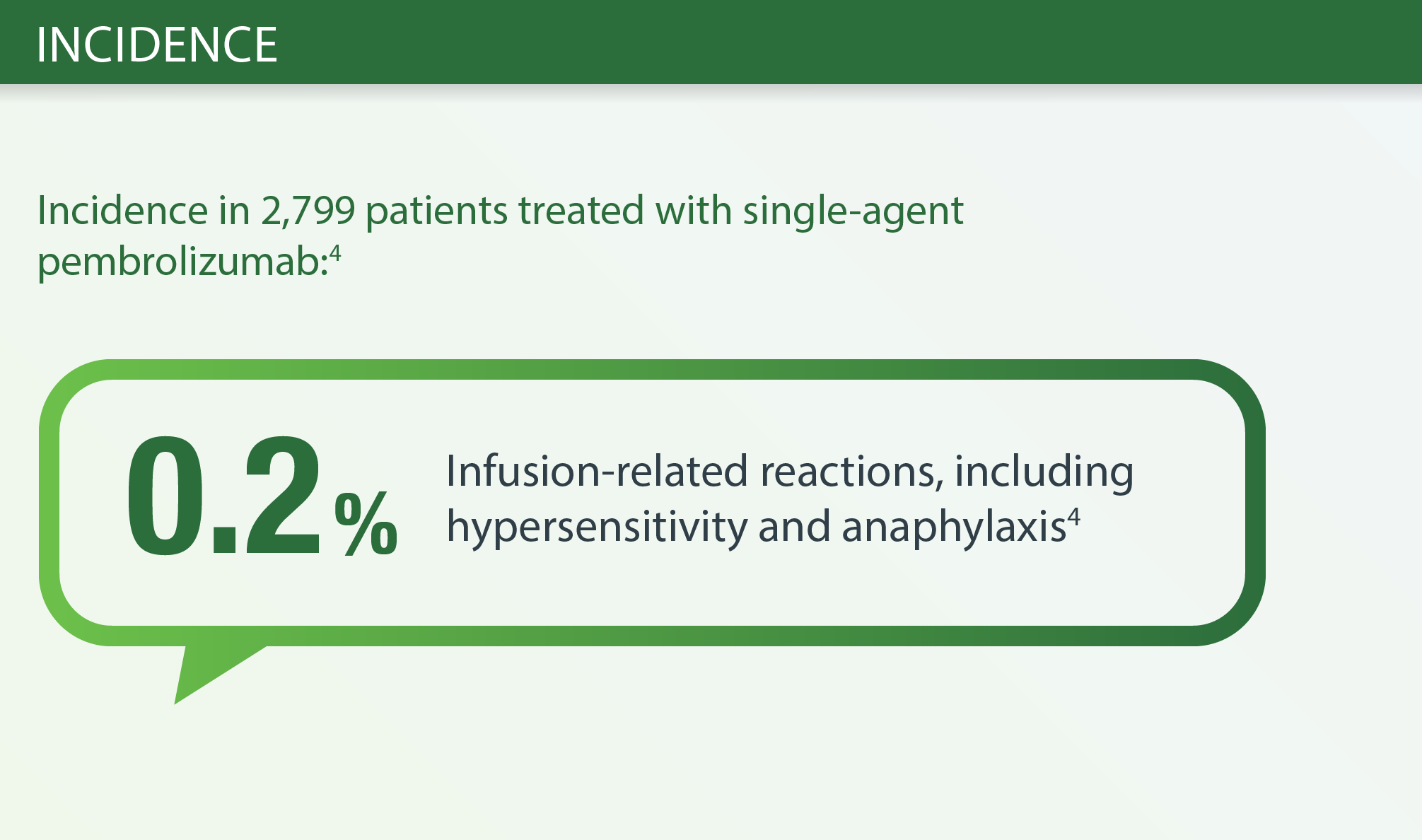
Infusion-related Reactions PEMBROLIZUMAB INCEDENCE
References:
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Accessed at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Last accessed: 15-1-2023.
- NCCN (National Comprehensive Cancer Network) V.1.2023. Accessed at: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Last accessed: 1-5-2023.
- Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. Journal of clinical oncology. 2023;39(36):4073-126.
- Egyptian drug authority KEYTRUDA® leaflet approval date: 23/05/2023.
MSD Egypt, 67 – El Tesseen St., Address Building, Fifth Settlement, New Cairo.
In case you need any updates or you have an inquiry or need to report on an adverse reaction, you can contact:
Veeva Code: EG-KEY-00280
Expiration Date: 10/07/2024





