Gastrointestinal – Hepatitis

OVERVIEW
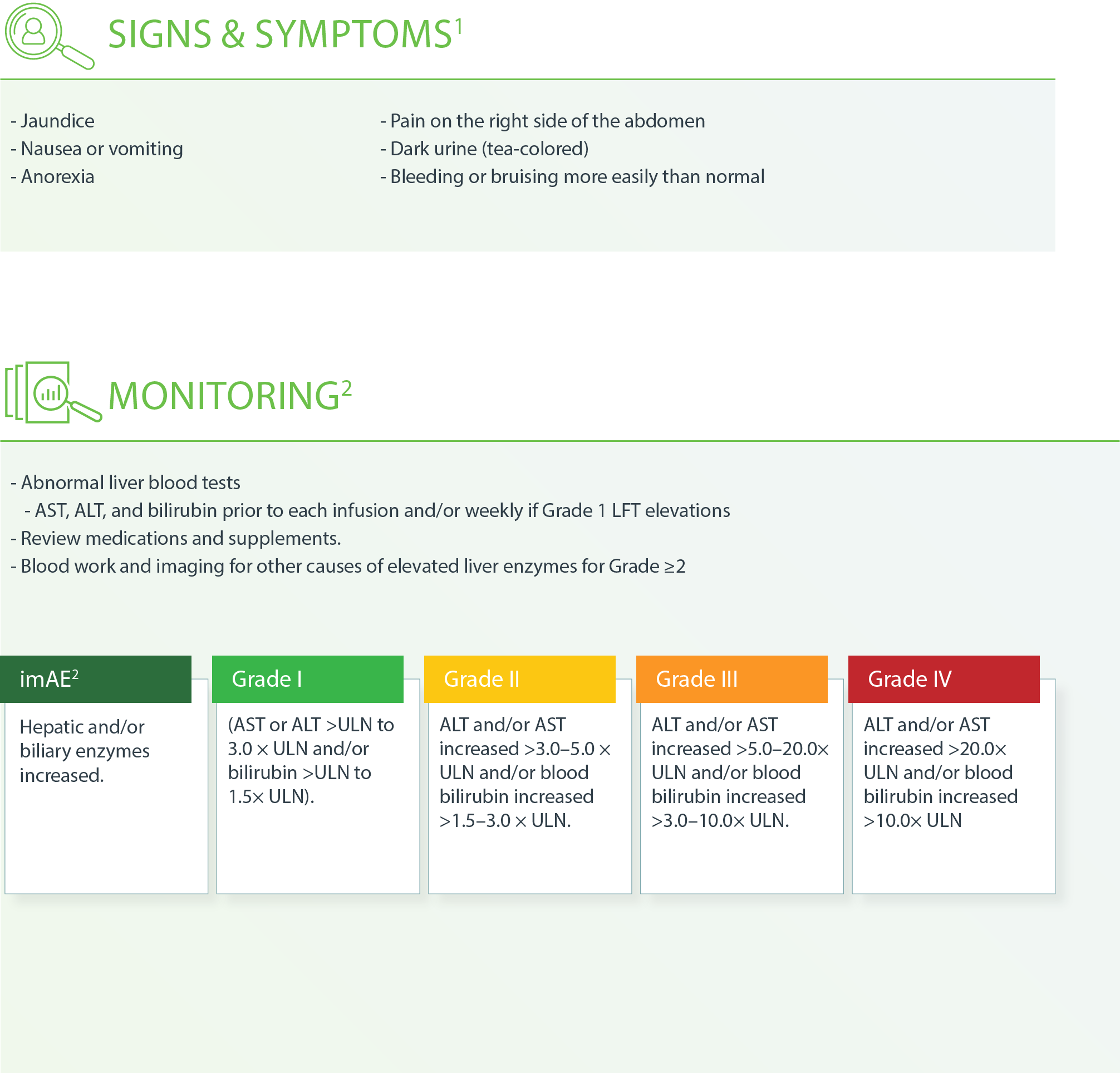
GUIDELINES
Gastrointestinal – Hepatitis
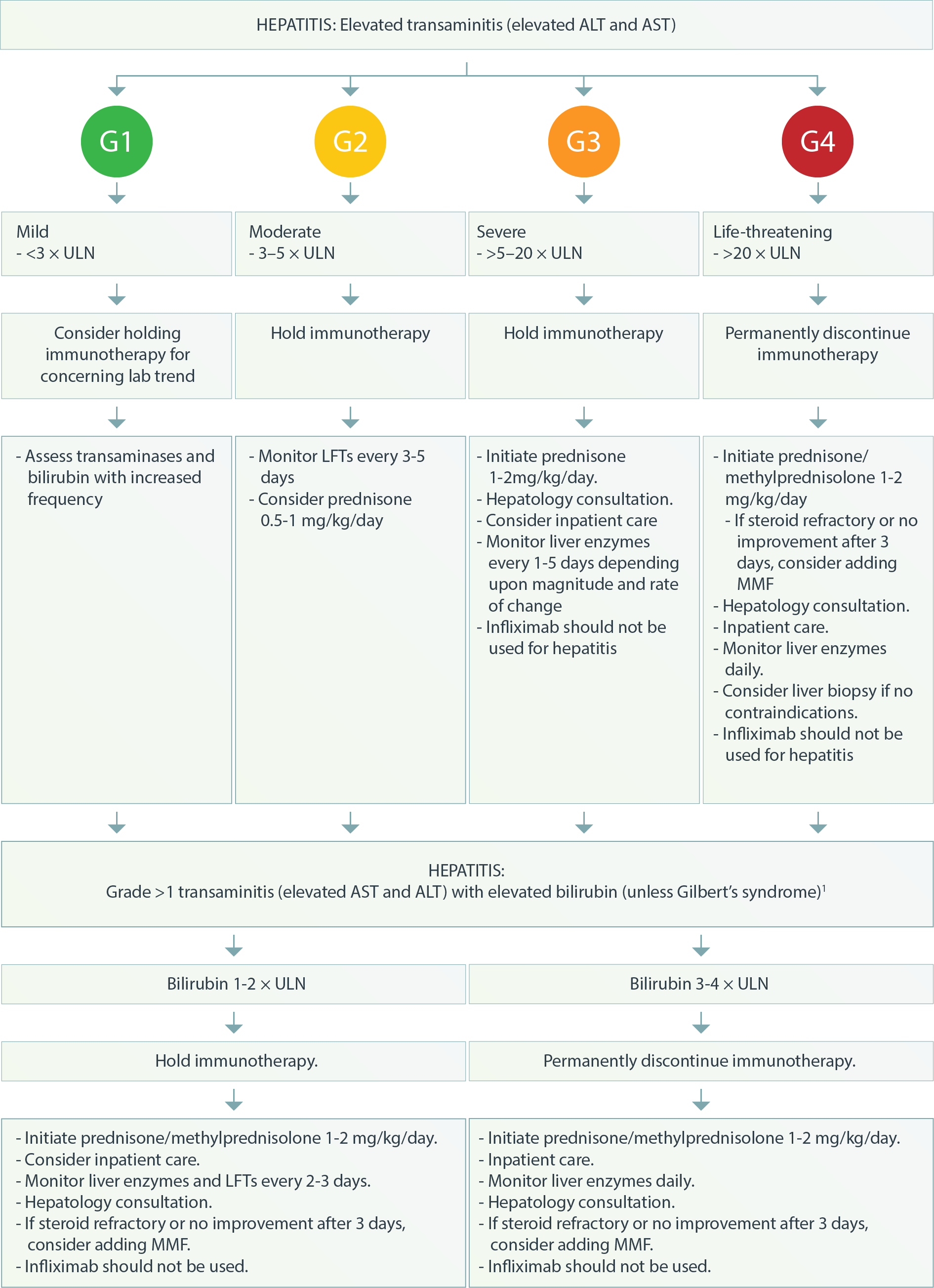
Gastrointestinal – Hepatitis
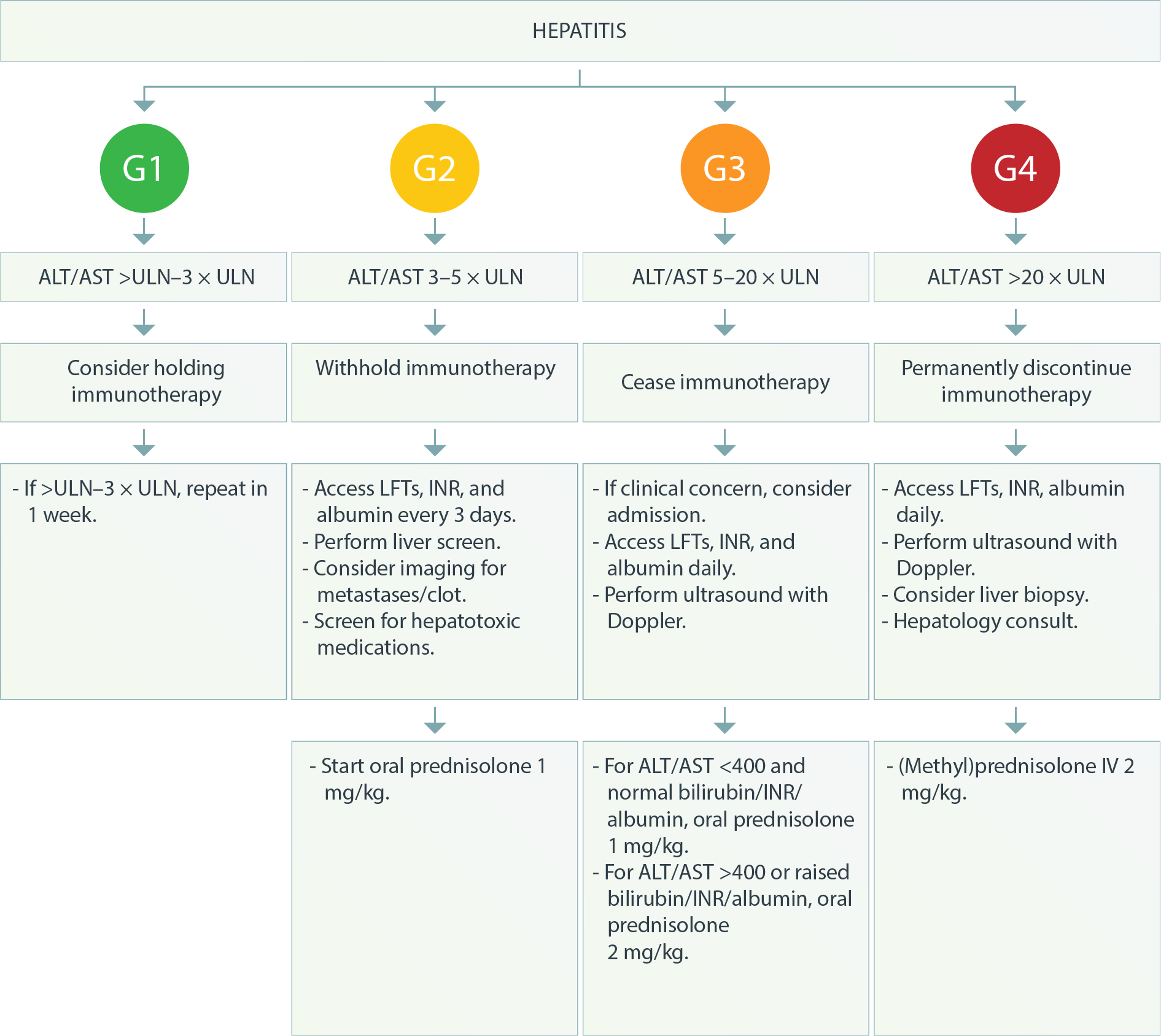
ALT: Alanine transaminase; ANA: Antinuclear antibodies; AST: Aspartate transaminase; LFTs: liver function tests; INR: International normalized ratio of prothrombin time; ULN: Upper limit of normal; US: Ultrasound.
Gastrointestinal – Hepatitis
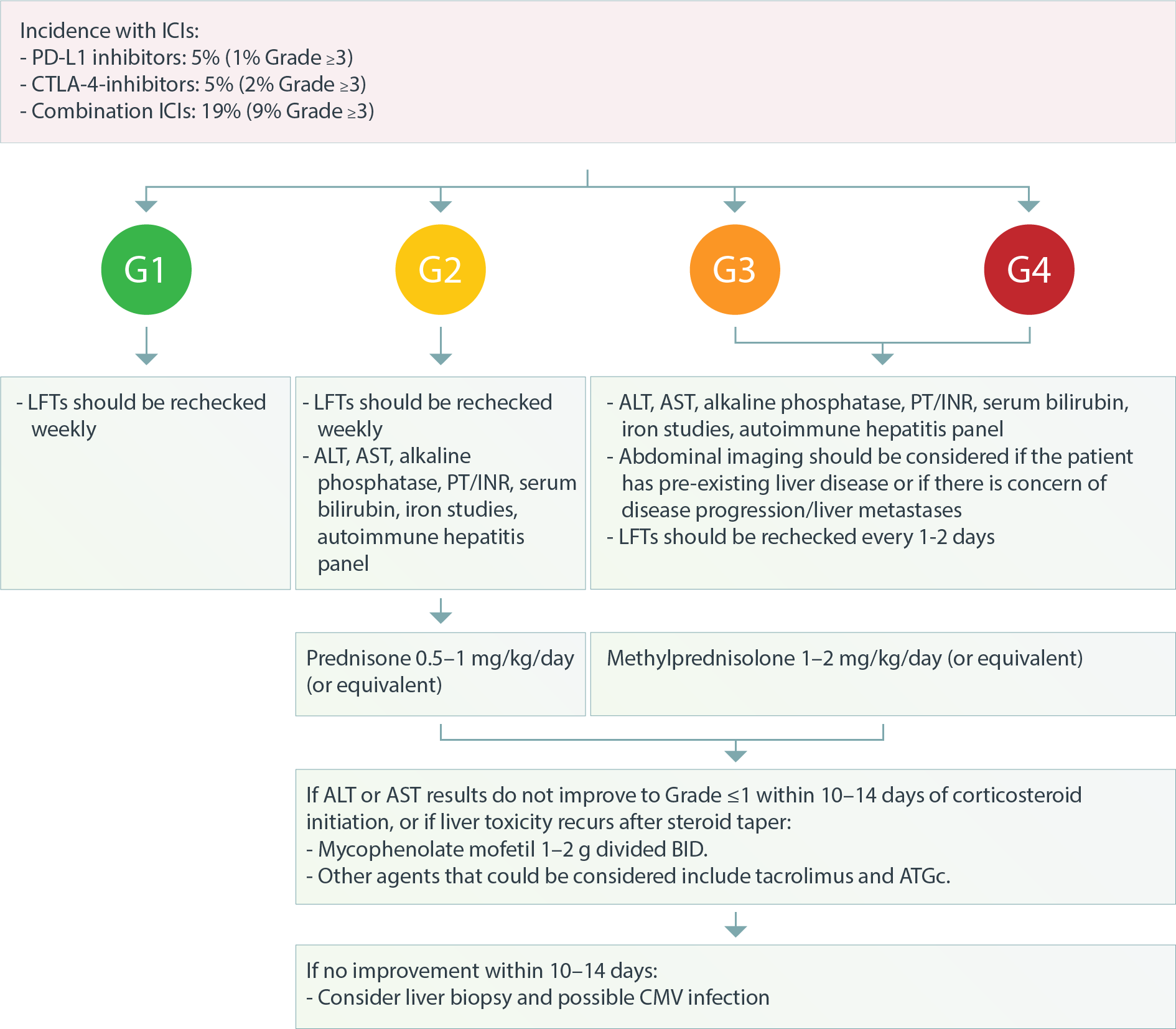
INCIDENCE
Gastrointestinal – Hepatitis

INCIDENCE
Gastrointestinal – Hepatitis
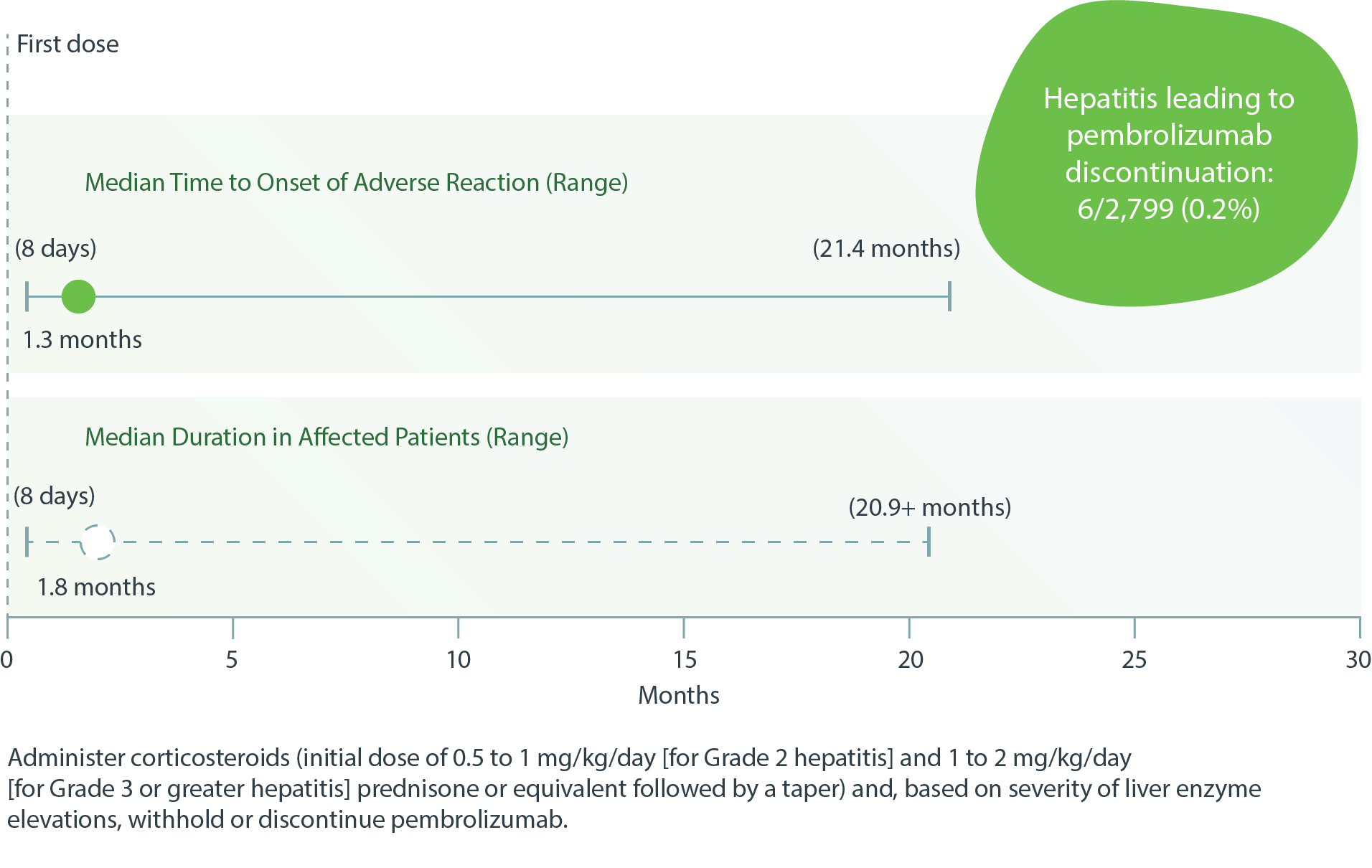
IMMUNE-RELATED HEPATITIS IN PATIENTS TREATED WITH PEMBROLIZUMAB (19/2,799)
References:
- NCCN (National Comprehensive Cancer Network) V.1.2023. Accessed at: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Last accessed: 1-5-2023
- Schneider J, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. Journal of clinical oncology. 2023; 39:4073-4126.
- Haanen , Obeid M, Spain L, Carbonnel F, Wang Y, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Annals of Oncology. 2022;33(12):1217-38
- Ahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. Journal for immunotherapy of cancer. 2021;9(6).
- Egyptian drug authority KEYTRUDA® leaflet approval date: 23/05/2023.
MSD Egypt, 67 – El Tesseen St., Address Building, Fifth Settlement, New Cairo.
In case you need any updates or you have an inquiry or need to report on an adverse reaction, you can contact:
Veeva Code: EG-KEY-00280
Expiration Date: 07/06/2024





