ICI Exposure and imAEs
General Considerations
ICI Exposure and imAEs

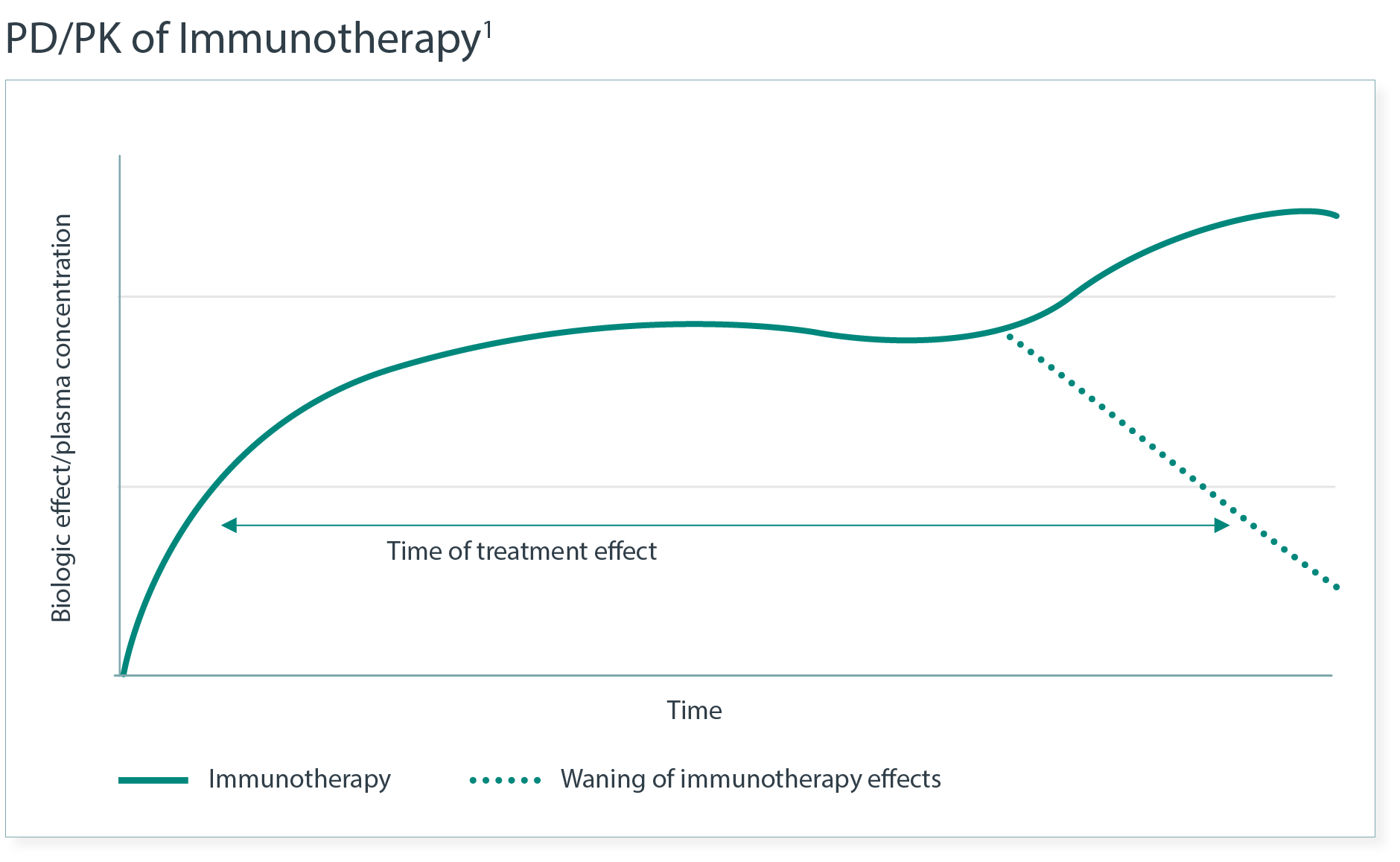
imAEs from immunotherapy can have a delayed onset and prolonged duration, in part due to PK/PD differences. 1
- Moreover, the relationship between imAEs and dose/exposure remains to be fully established.

imAE risk can vary with ICI dose.
- imAE risk appears to be dose independent with PD-1 inhibitors.2
- Risk appears to be dose dependent with CTLA-4 inhibitors.3
References:
- Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, Hamad L, et al Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017 Nov 21;5(1):95
- Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019 Sep;16(9):563-580.
- Wu J, Hong D, Zhang X, Lu X, Miao J. PD-1 inhibitors increase the incidence and risk of pneumonitis in cancer patients in a dose-independent manner: a meta-analysis. Sci Rep. 2017 Mar 8;7:44173
MSD Egypt, 67 – El Tesseen St., Address Building, Fifth Settlement, New Cairo.
In case you need any updates or you have an inquiry or need to report on an adverse reaction, you can contact:
Veeva Code: EG-KEY-00280
Expiration Date: 10/07/2024





