Cardiovascular System

o

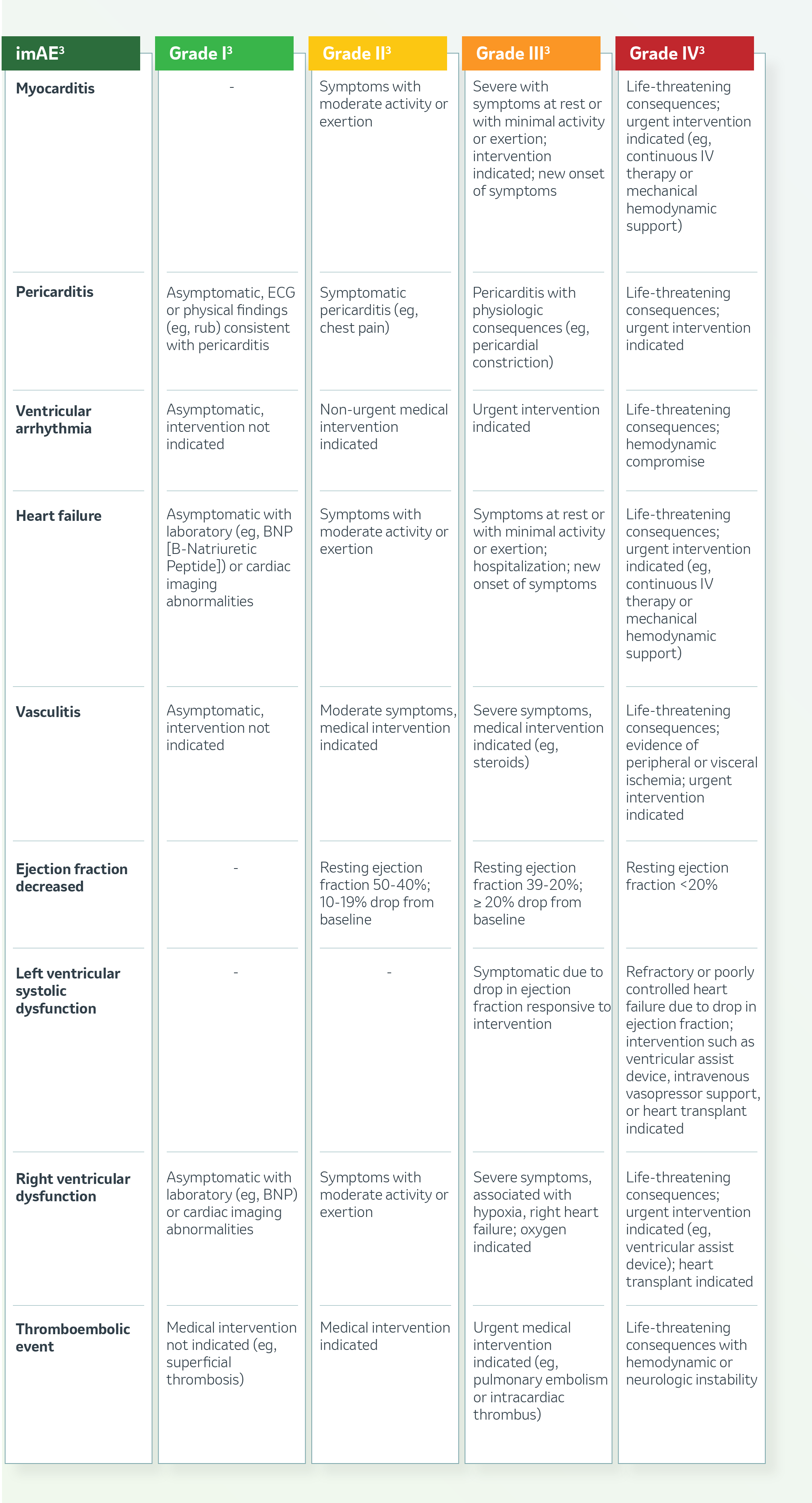
c To assess for associated myositis
nonsteroidal anti-inflammatory drugs [NSAIDs], narcotics, intravenous [IV] fluids); prophylactic medications indicated for 24 hours.
d Lipid panel would be recommended at baseline to assess CV risk. Also consider troponin and NTproBNP at baseline for identifying those at increased risk. Also, consider high-sensitivity troponin and NTproBNP at baseline and serially during treatment to detect abnormal blood biomarkers that may precede symptomatic myocarditis induced by ICI.
e Consider ESR, CRP, or other inflammatory markers
f Use of multiparameter tissue characterization by MRI, including T1 and T2 mapping and application of modified Lake Louise Criteria provides important diagnostic value for myocarditis. If cardiac MRI is negative or myocarditis is highly suspected, consider endomyocardial biops
GUIDELINES
Cardiovascular – Pericarditis/pericardial effusion/Myocarditis 2
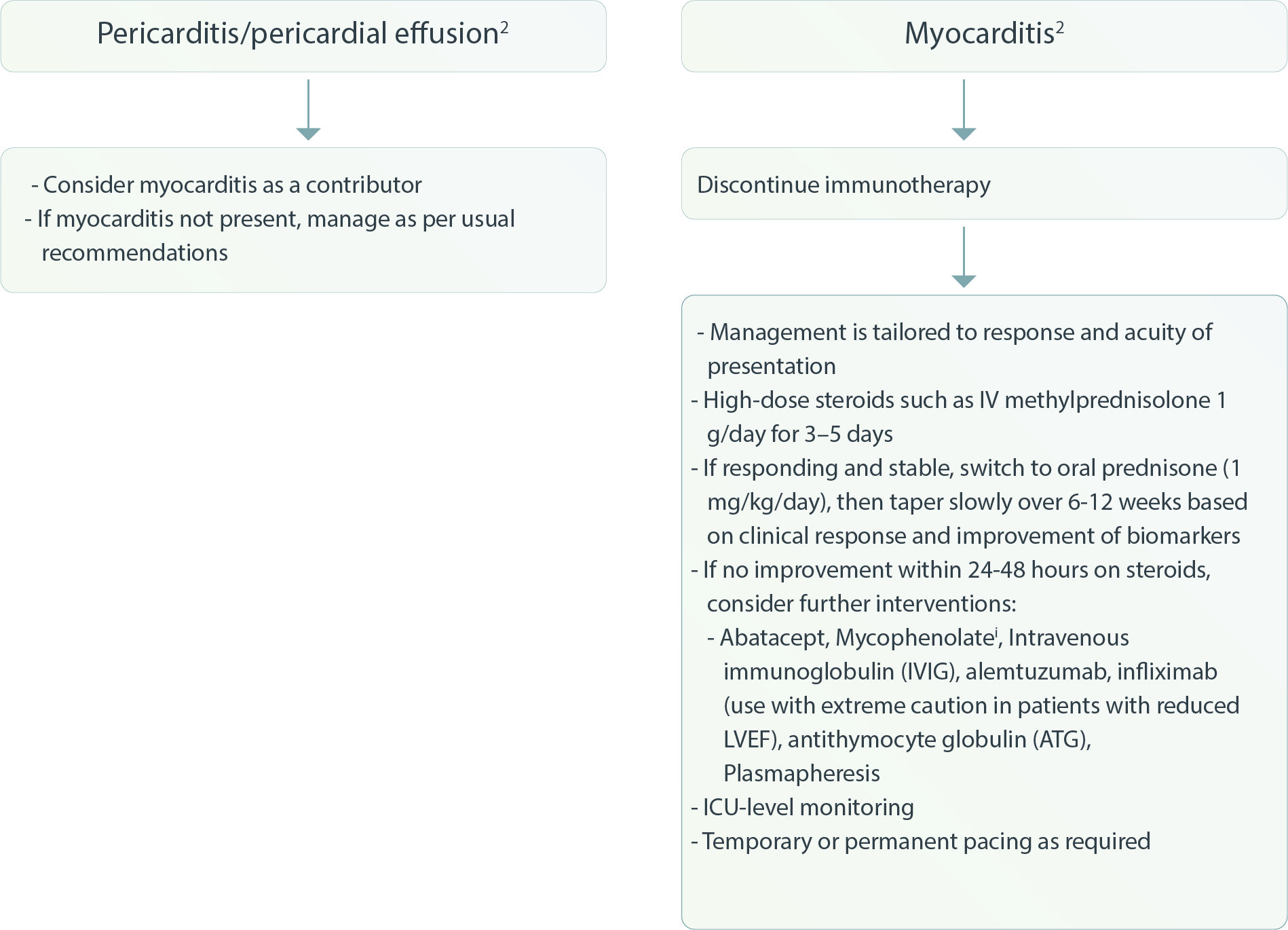
i Mycophenolate mofetil treatment (0.5–1 g every 12 h).
Cardiovascular – Venous Thromboembolism1
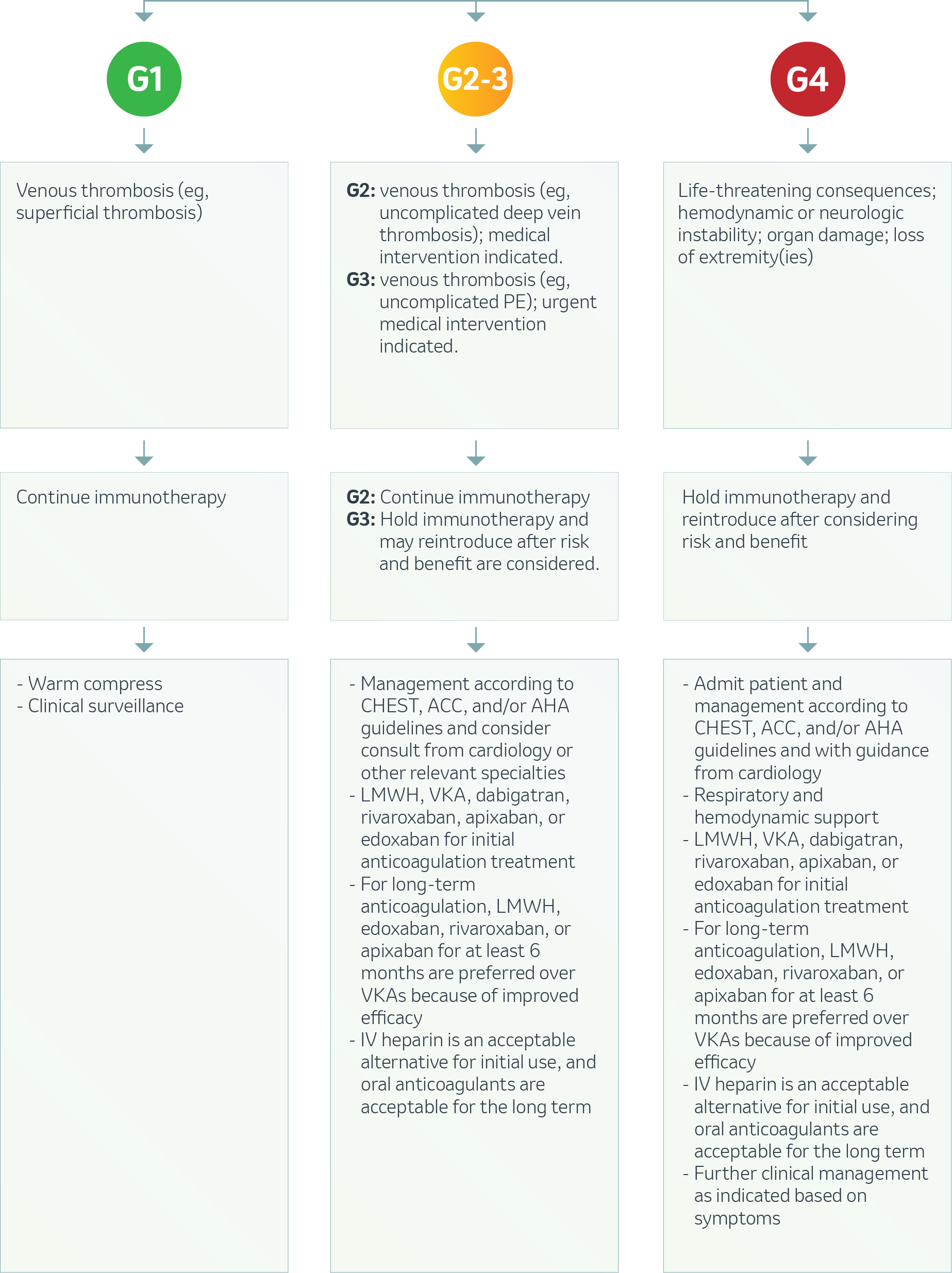
ACC: American College of Cardiology; AHA: American Heart Association; LMWH: Low molecular weight heparin; VKA: Vitamin K antagonist
Cardiovascular – Myocarditis, Pericarditis, Arrhythmias, Conduction Abnormalities, Impaired Ventricular Function With Heart Failure, and Vasculitis1
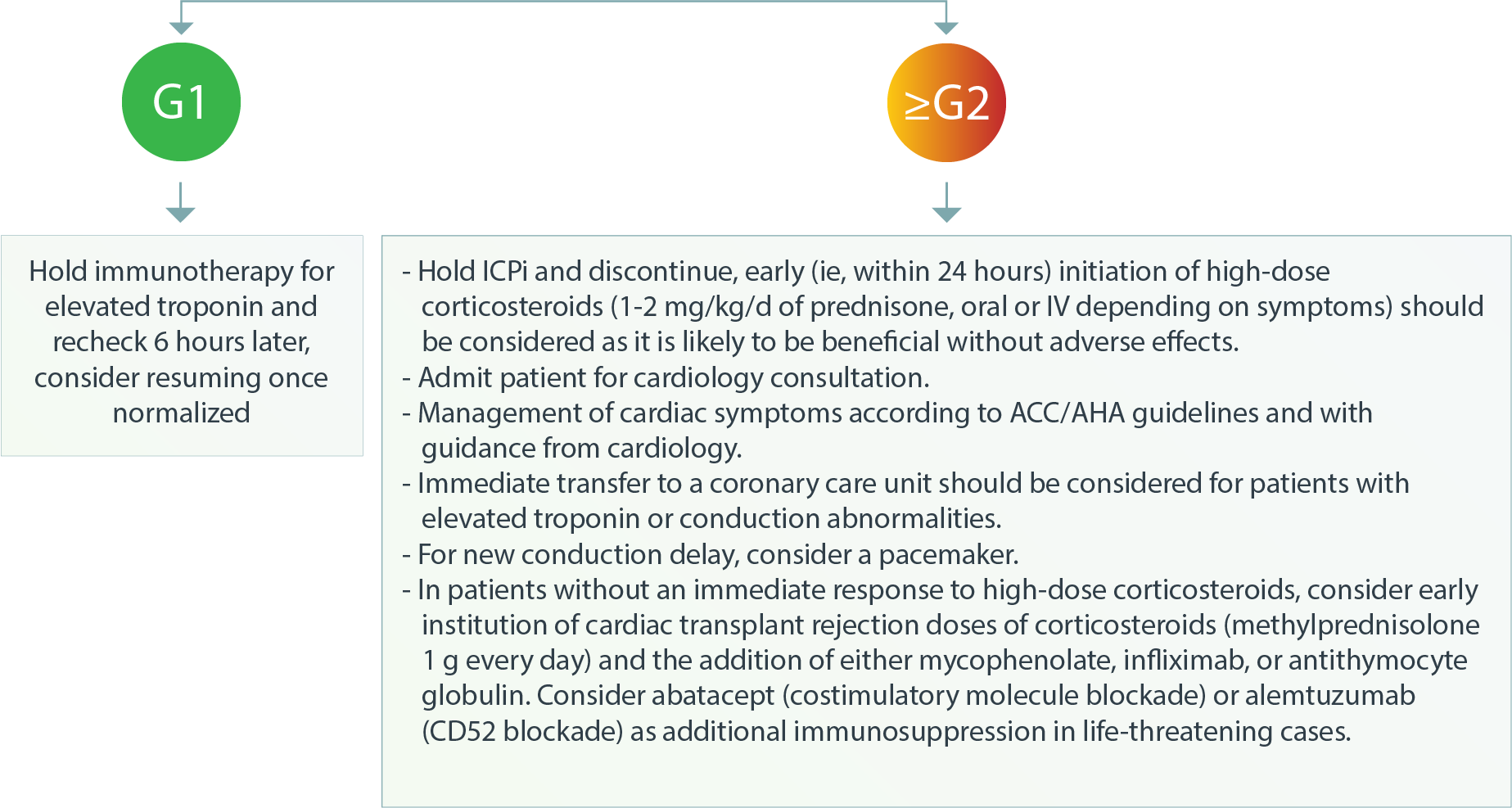
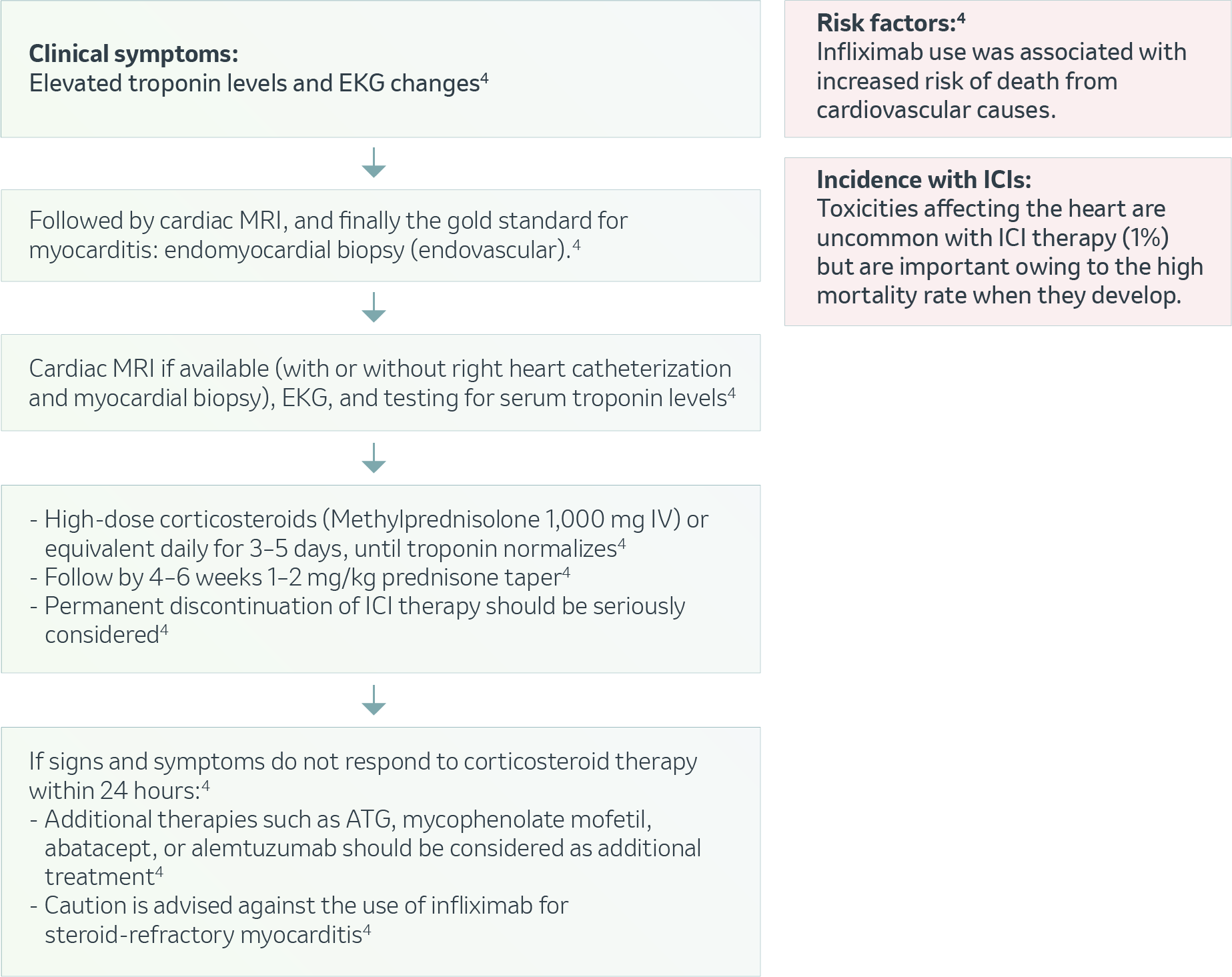
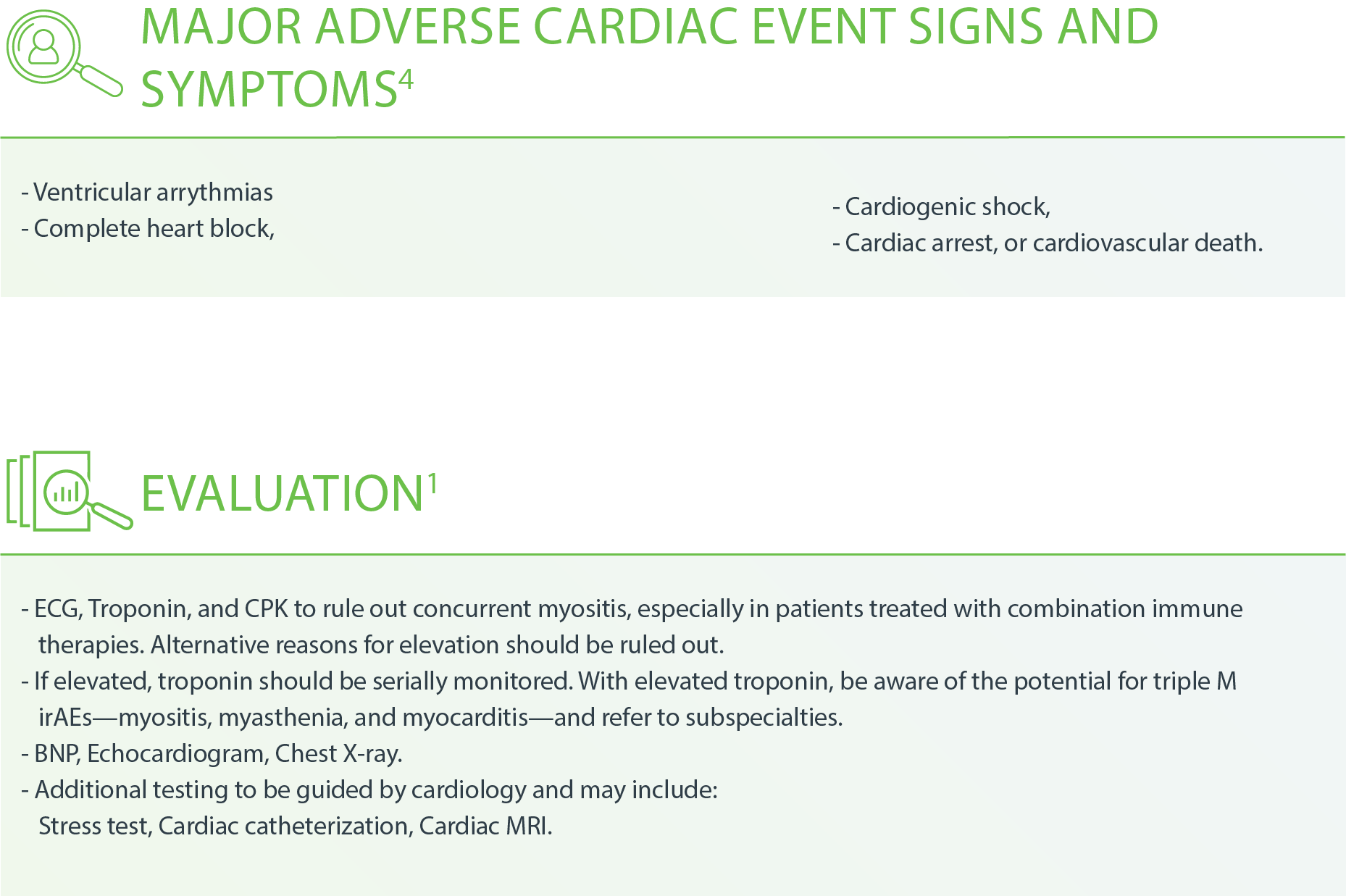
Cardiovascular – Myocarditis4
ADDITIONAL EMERGENCY SETTING INFORMATION
Cardiovascular
References:
- Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. Journal of clinical oncology. 2023;39(36):4073-126
- NCCN (National Comprehensive Cancer Network) V.1.2023. Accessed at: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Last accessed: 1-5-2023.
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Accessed at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Last accessed: 15-1-2023.
- Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. Journal for immunotherapy of cancer. 2021;9(6)
MSD Egypt, 67 – El Tesseen St., Address Building, Fifth Settlement, New Cairo.
In case you need any updates or you have an inquiry or need to report on an adverse reaction, you can contact:
Veeva Code: EG-KEY-00280
Expiration Date: 10/07/2024





