Dermatologic Toxicity

OVERVIEW

GUIDELINES
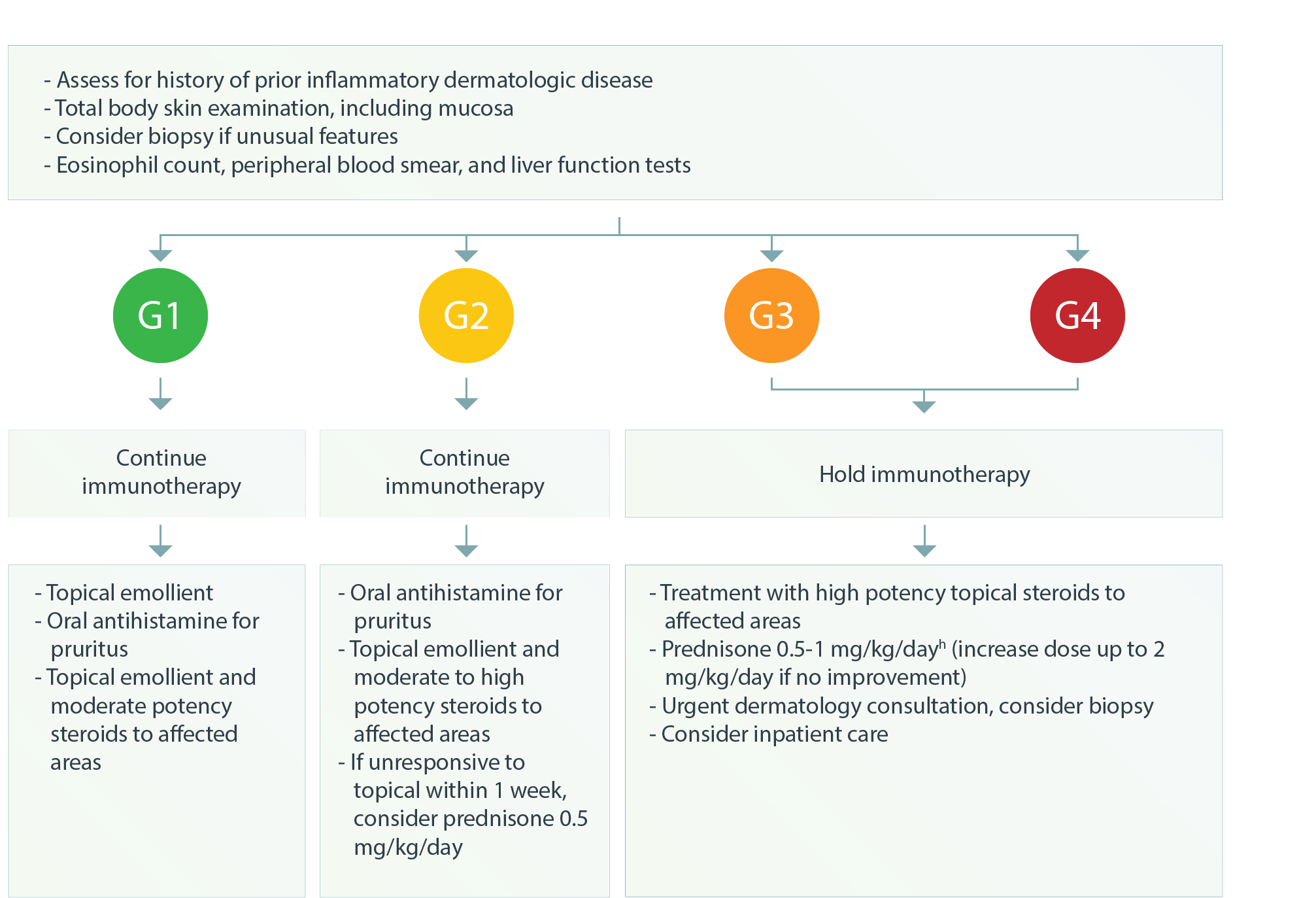
Dermatologic – Maculopapular Rash1
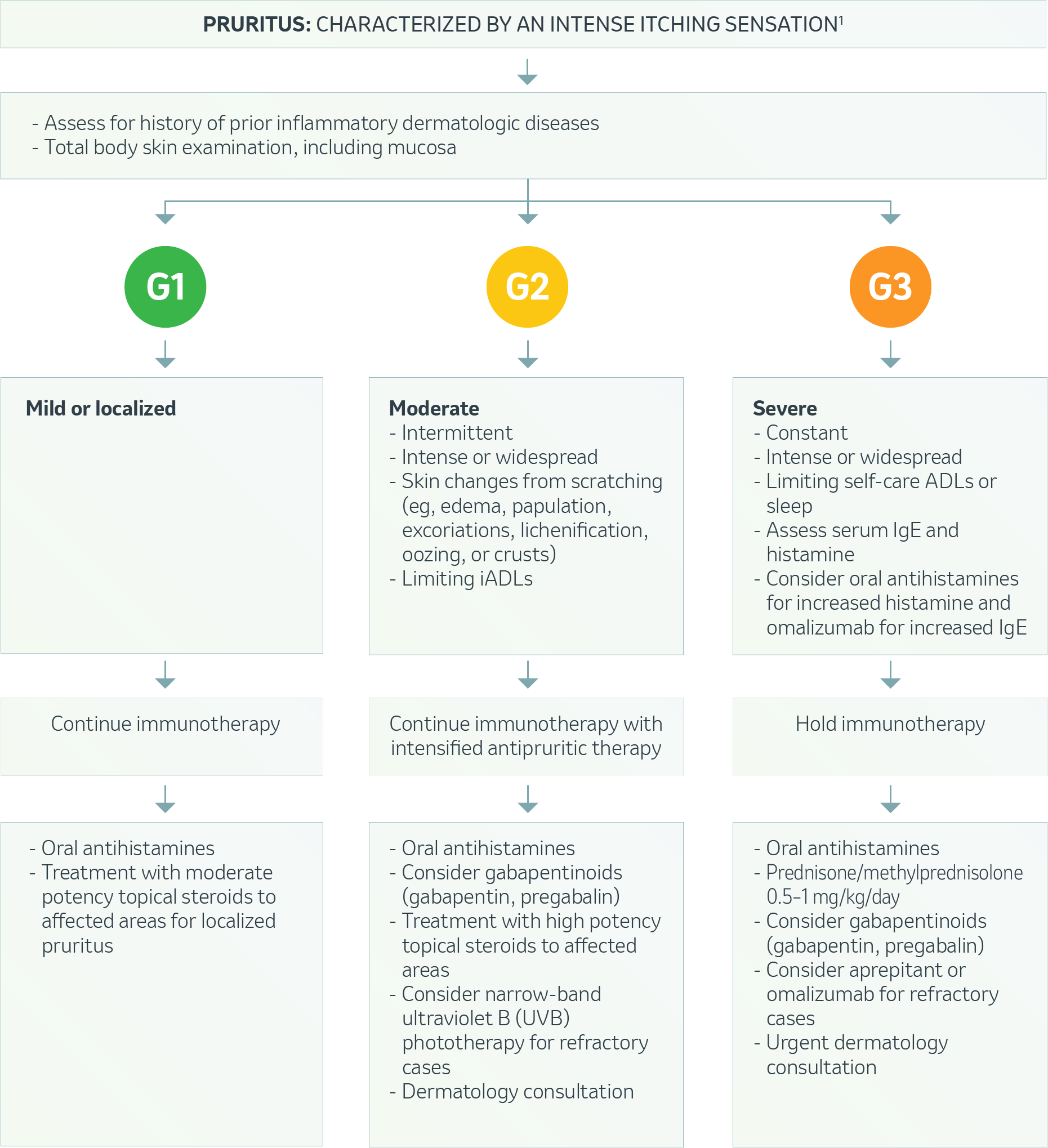
Dermatologic – Pruritus1
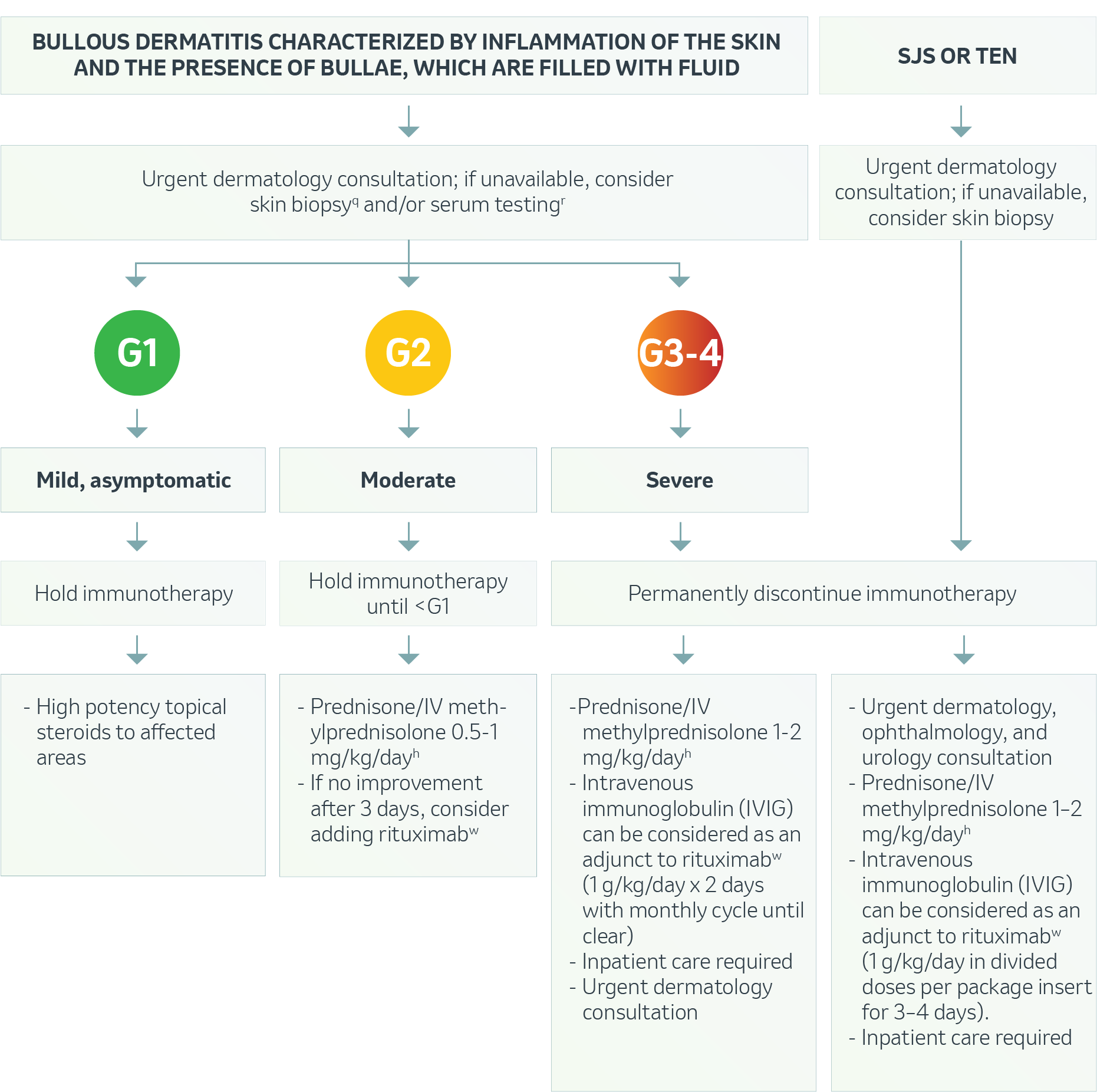
Dermatologic – Bullous Dermatitis and SJS/TEN1
b These features can be used to assist with the diagnosis of DRESS (drug rash with eosinophilia and systemic symptoms) syndrome. This syndrome is typically characterized by a maculopapular rash that involves the face and ears and typically presents with swelling of the face and hand.
m Consider holding in select cases
q Skin biopsies may also be performed on peripheral bullae intact skin. Two biopsies should be performed with one being sent for direct immunofluorescence testing in normal saline.
r The following serologic tests may be considered for autoimmune/imAEs-associated bullous disorders: bullous pemphigoid antibodies, desmoglein 1,3 (pemphigus) antibodies, anti-skin antibody or indirect immunofluorescence.
h Treat until symptoms improve to Grade 1, then taper over 4–6 weeks.
w 1000 mg once every 2 weeks for 2 doses (in combination with a tapering course of glucocorticoids), followed by maintenance of rituximab 500 mg at months 12 and 18 as needed.
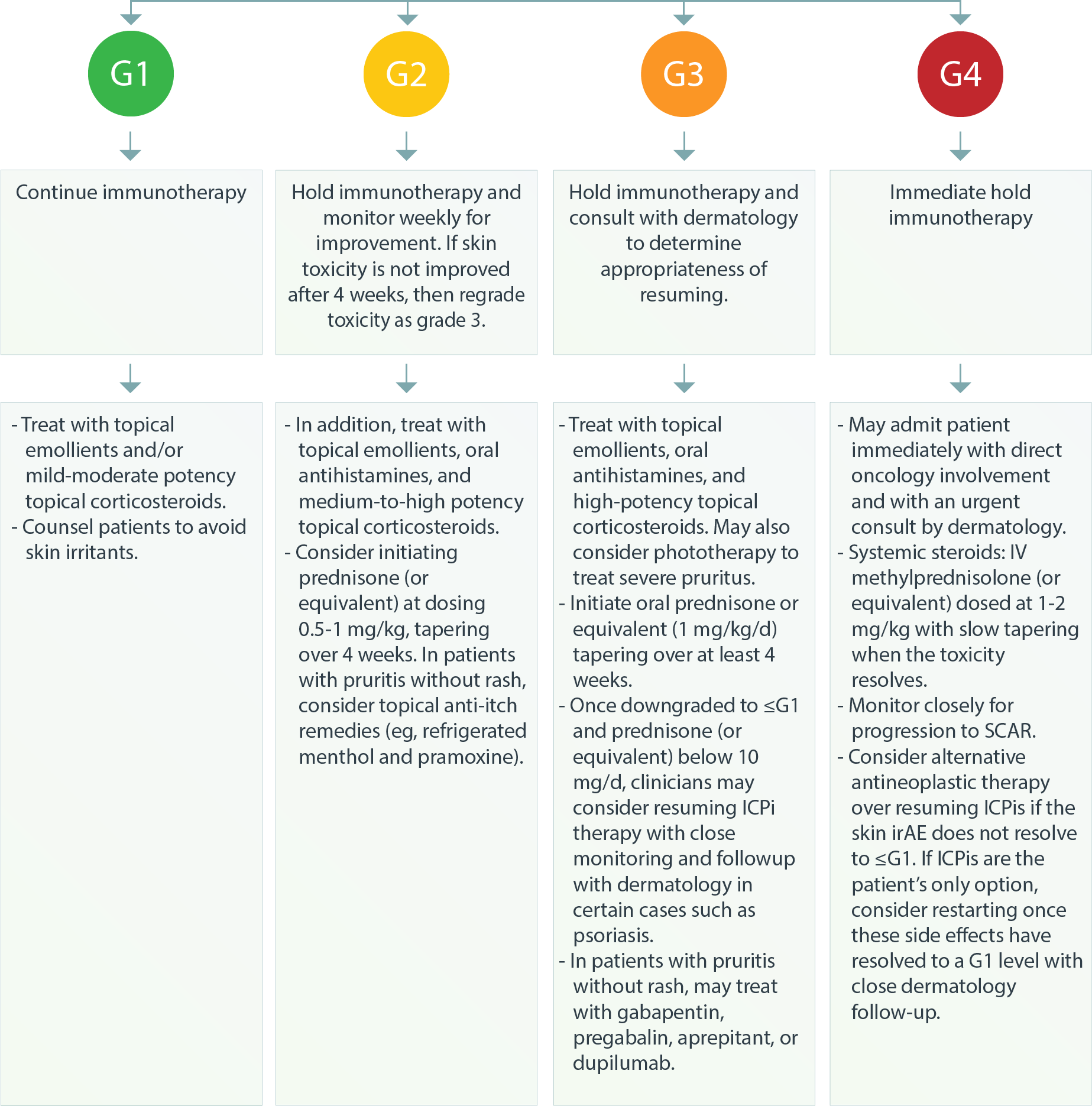
Dermatologic – Rash or Inflammatory Dermatitis3
Dermatologic – Bullous Dermatoses3
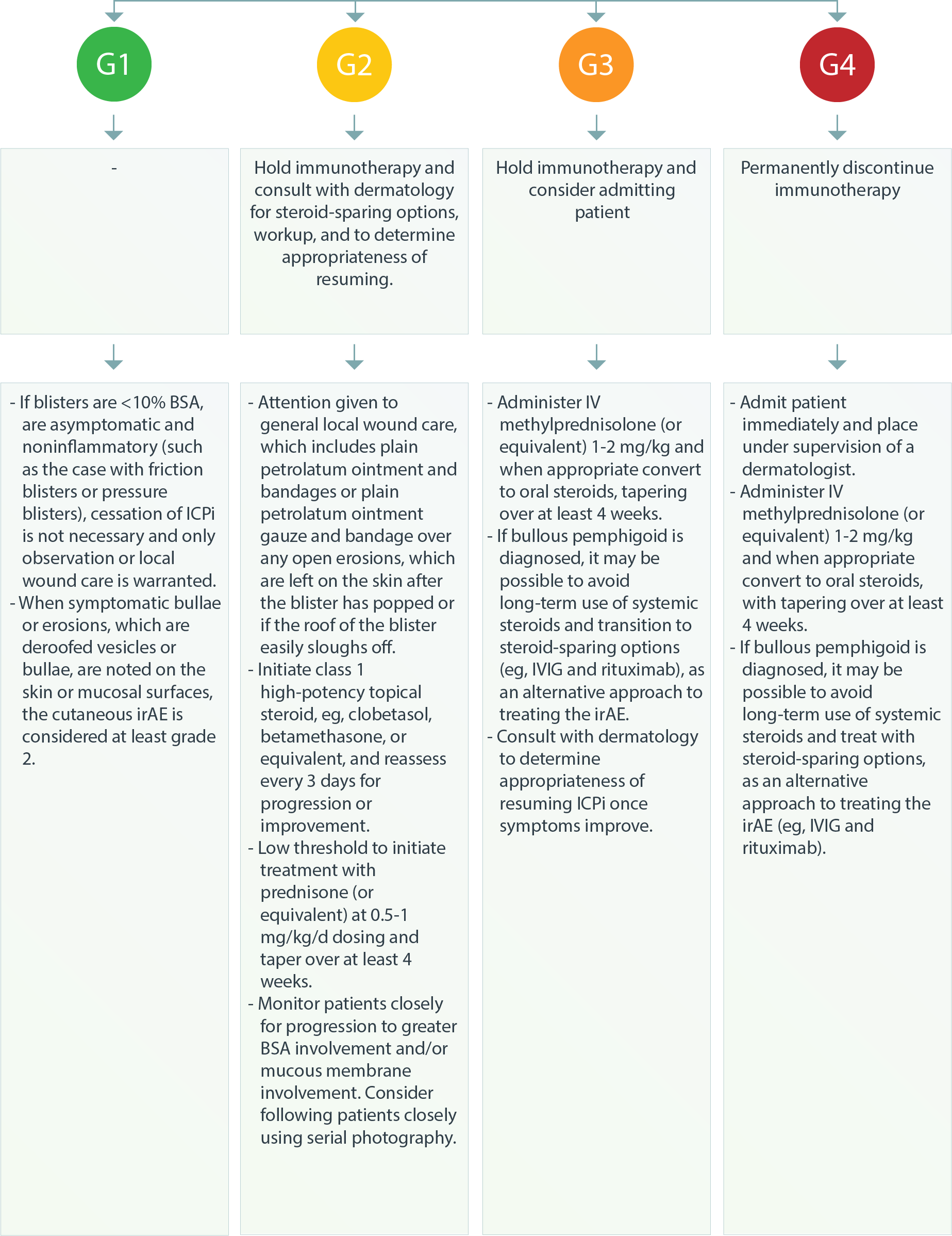
Dermatologic – SCAR3
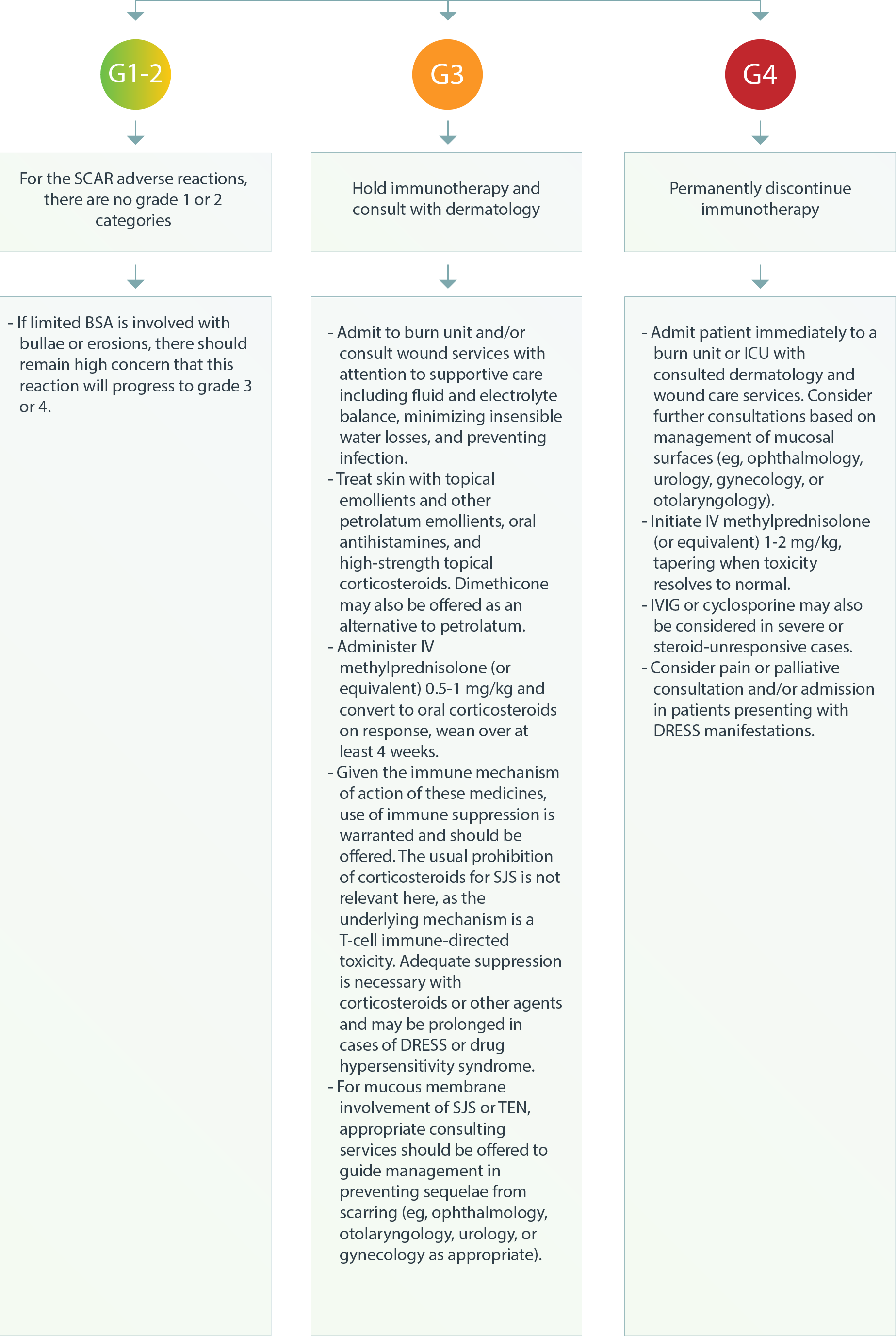
SCAR: Severe cutaneous adverse reactions; SJS/TEN: Stevens-Johnson syndrome/toxic epidermal necrolysis; DRESS: Drug reaction with eosinophilia and systemic symptom; IVIG: Intravenous immune globulin
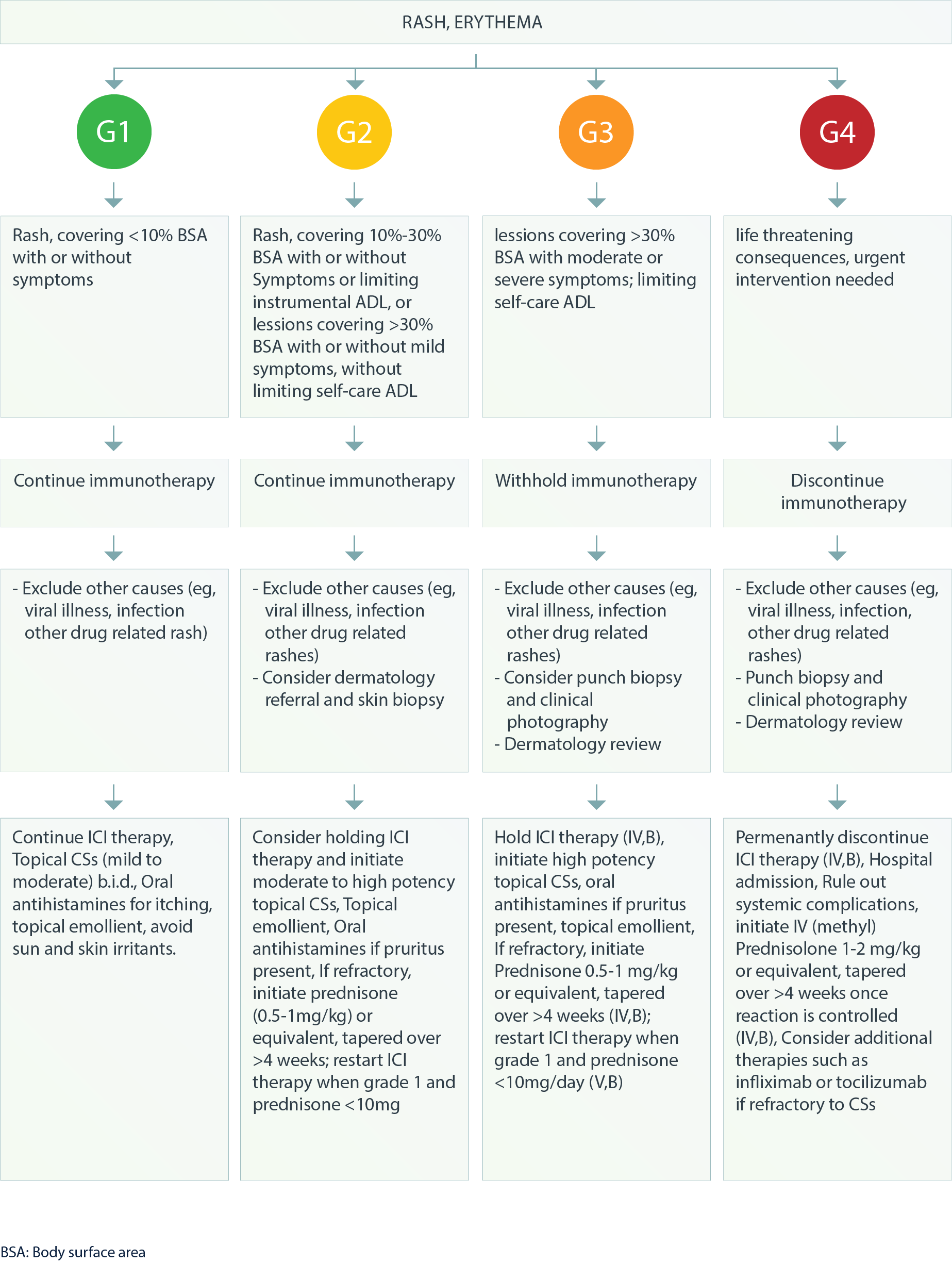
Dermatologic – Rash, Erythema4
Dermatologic – Rash & Pruritus5
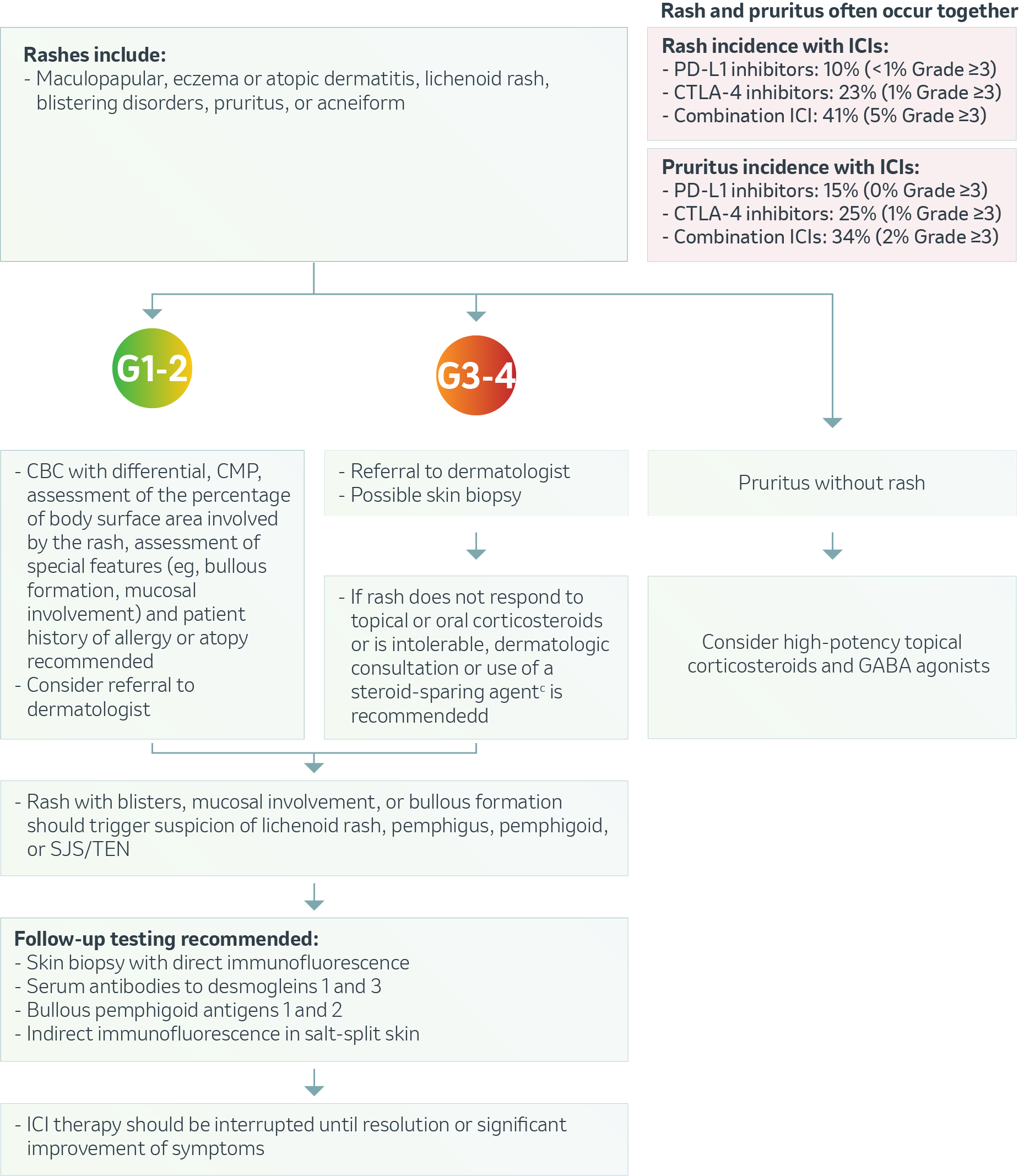
CTLA-4: Cytotoxic T-lymphocyte–associated protein 4; PD-1: Programmed cell death 1; ICI: Immune checkpoint inhibitors; CBC: Complete blood count; CMP: Comprehensive metabolic panel; GABA: γ-aminobutyric acid; SJS/TEN: Stevens-Johnson syndrome/toxic epidermal necrolysis
INCIDENCE
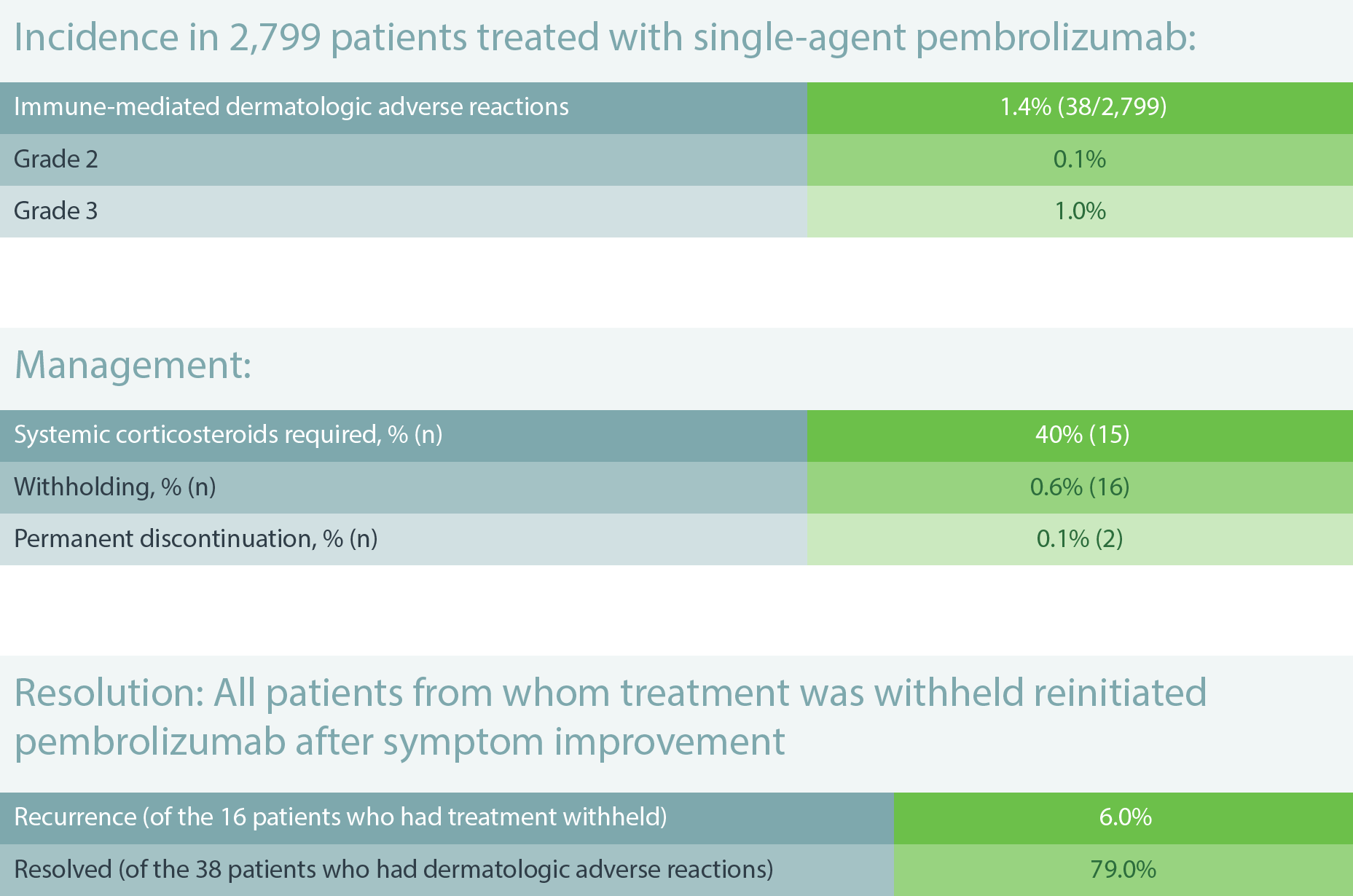
Immune-Mediated Dermatologic Adverse Reactions6
References:
- NCCN (National Comprehensive Cancer Network) V.1.2023. Accessed at: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Last accessed: 1-5-2023
- US Department of health and human services. Common terminology criteria for adverseevents (CTCAE) Version 5.0. Accessed at: Common Terminology Criteria for Adverse Events(CTCAE) (cancer.gov). Last Accessed: 15-1-2023.
- Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. Journal of clinical oncology. 2023;39(36):4073-126.
- Haanen JB, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Corrections to “Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2018;29:iv264-6.
- Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. Journal for immunotherapy of cancer. 2021;9(6)
- Egyptian drug authority KEYTRUDA® leaflet approval date: 23/05/2023.
MSD Egypt, 67 – El Tesseen St., Address Building, Fifth Settlement, New Cairo.
In case you need any updates or you have an inquiry or need to report on an adverse reaction, you can contact:
Veeva Code: EG-KEY-00280
Expiration Date: 10/07/2024





