Musculoskeletal Toxicity

OVERVIEW
GUIDELINES
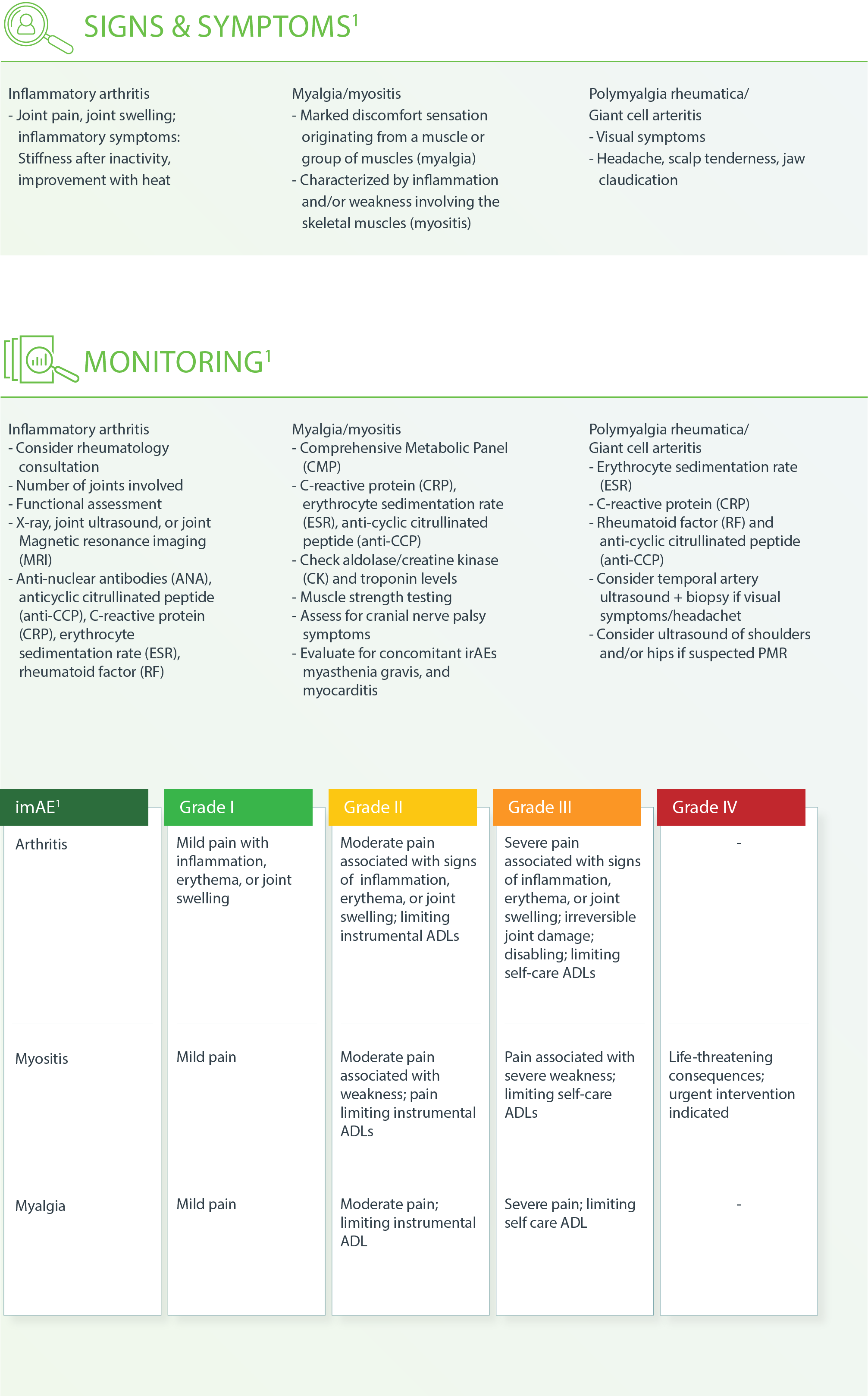
Musculoskeletal – Inflammatory Arthritis1
m Consider ESR, CRP to monitor response if elevated at the onset of therapy.
g Treat until symptoms improve to Grade ≤1, then taper over 4-6 weeks.
h If patients need to be on steroids long-term.
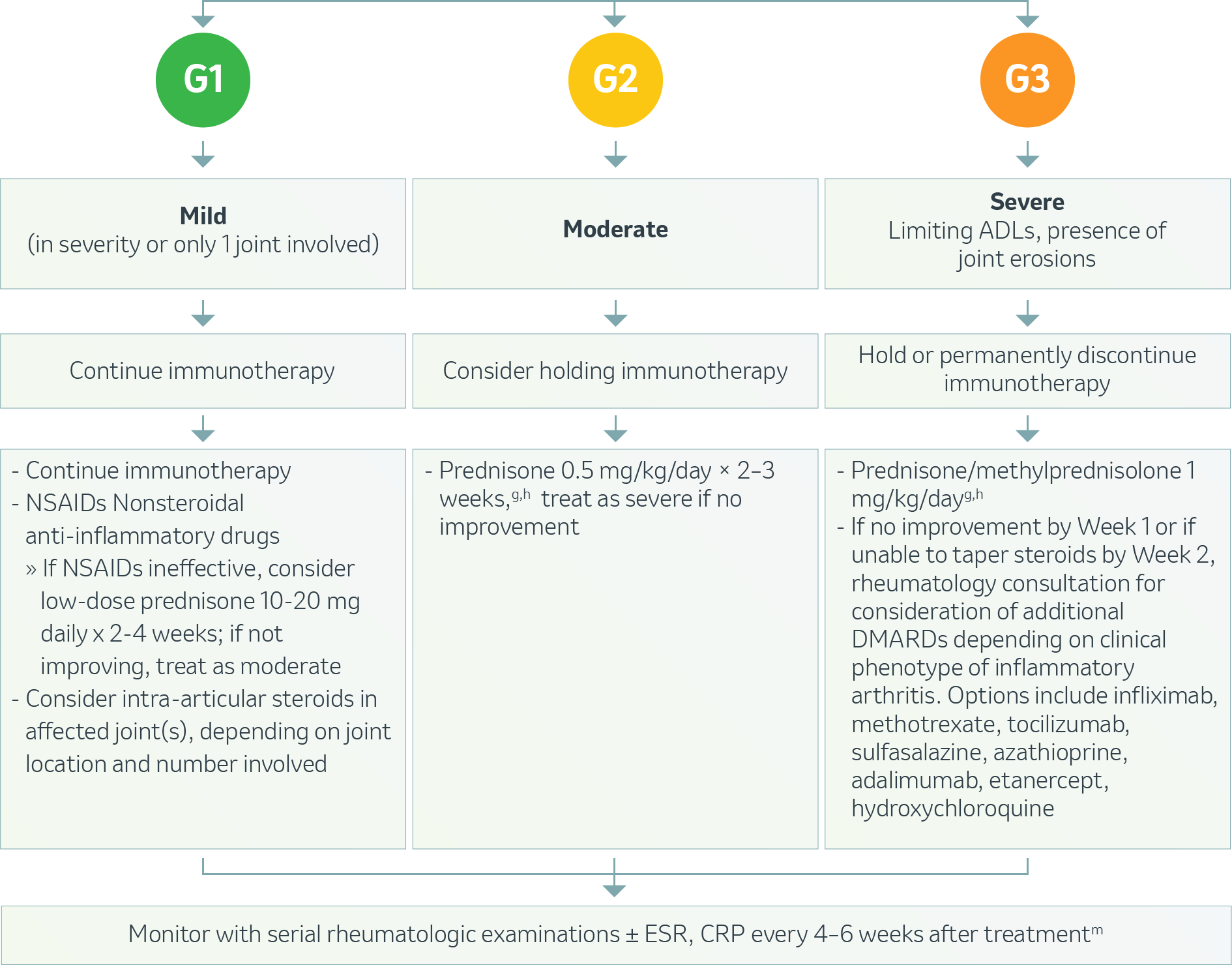
Musculoskeletal – Myalgia or Myositis1
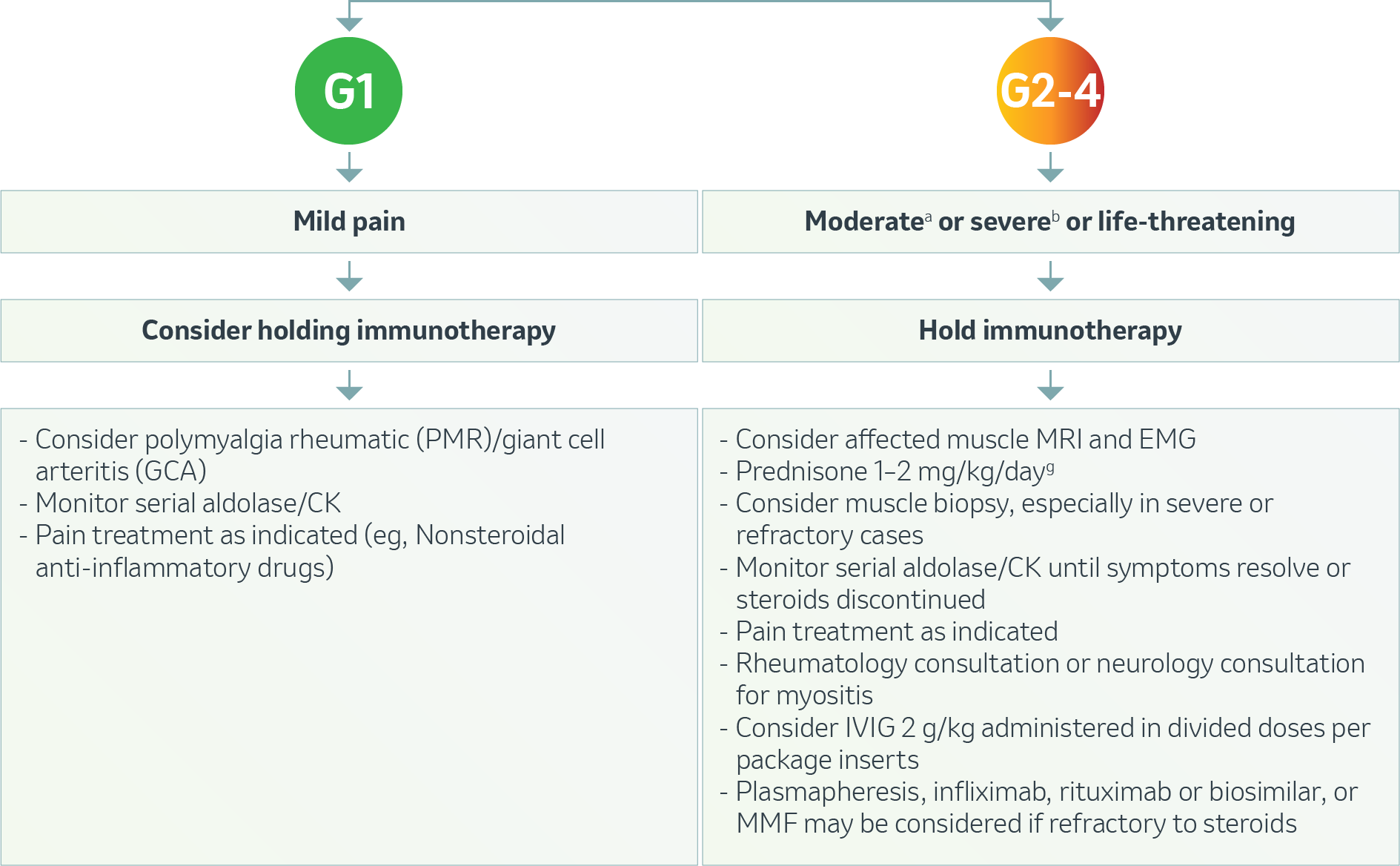
g Treat until symptoms improve to Grade ≤1, then taper over 4-6 weeks.
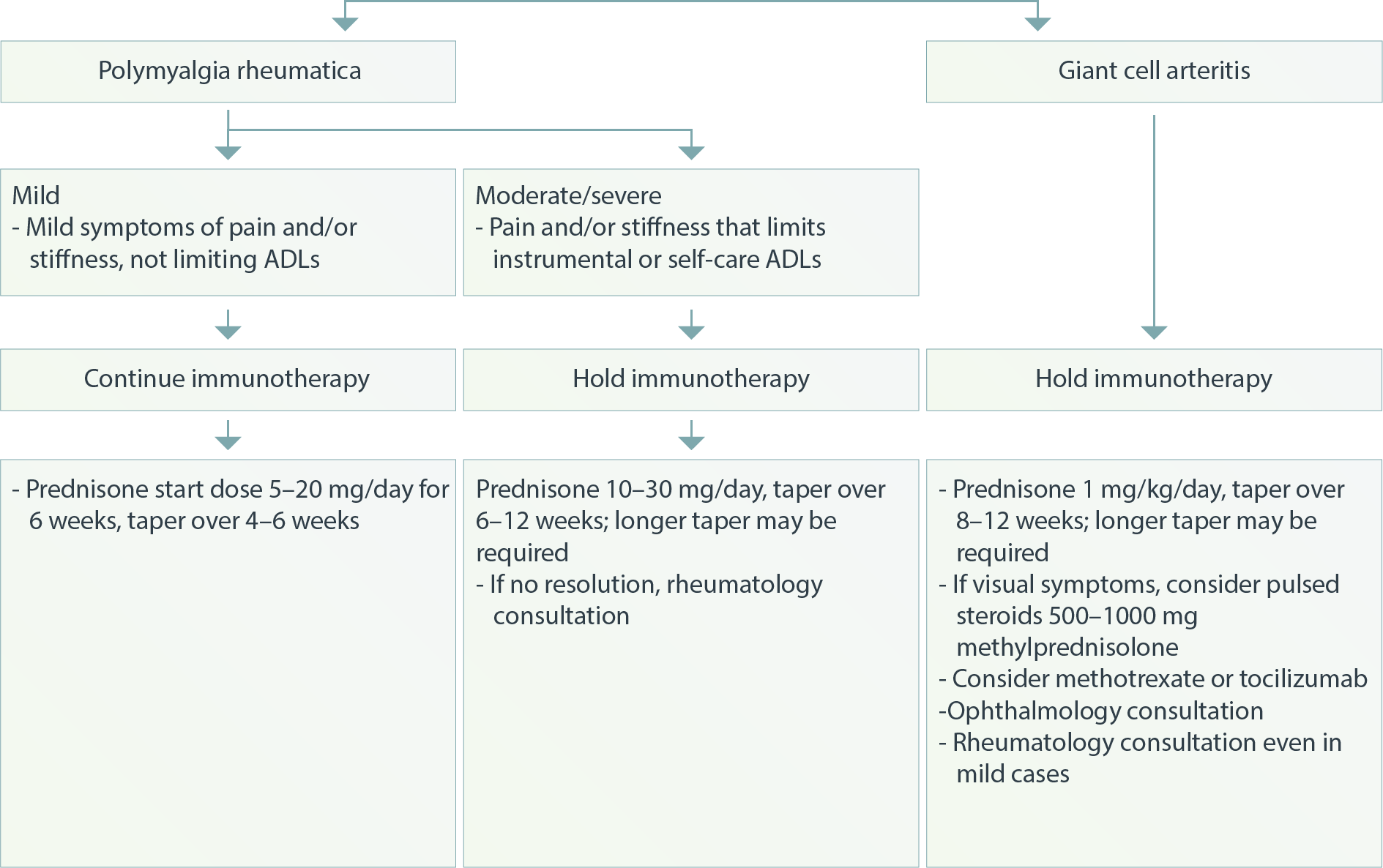
Musculoskeletal – Polymyalgia Rheumatica and Giant Cell Arteritis1
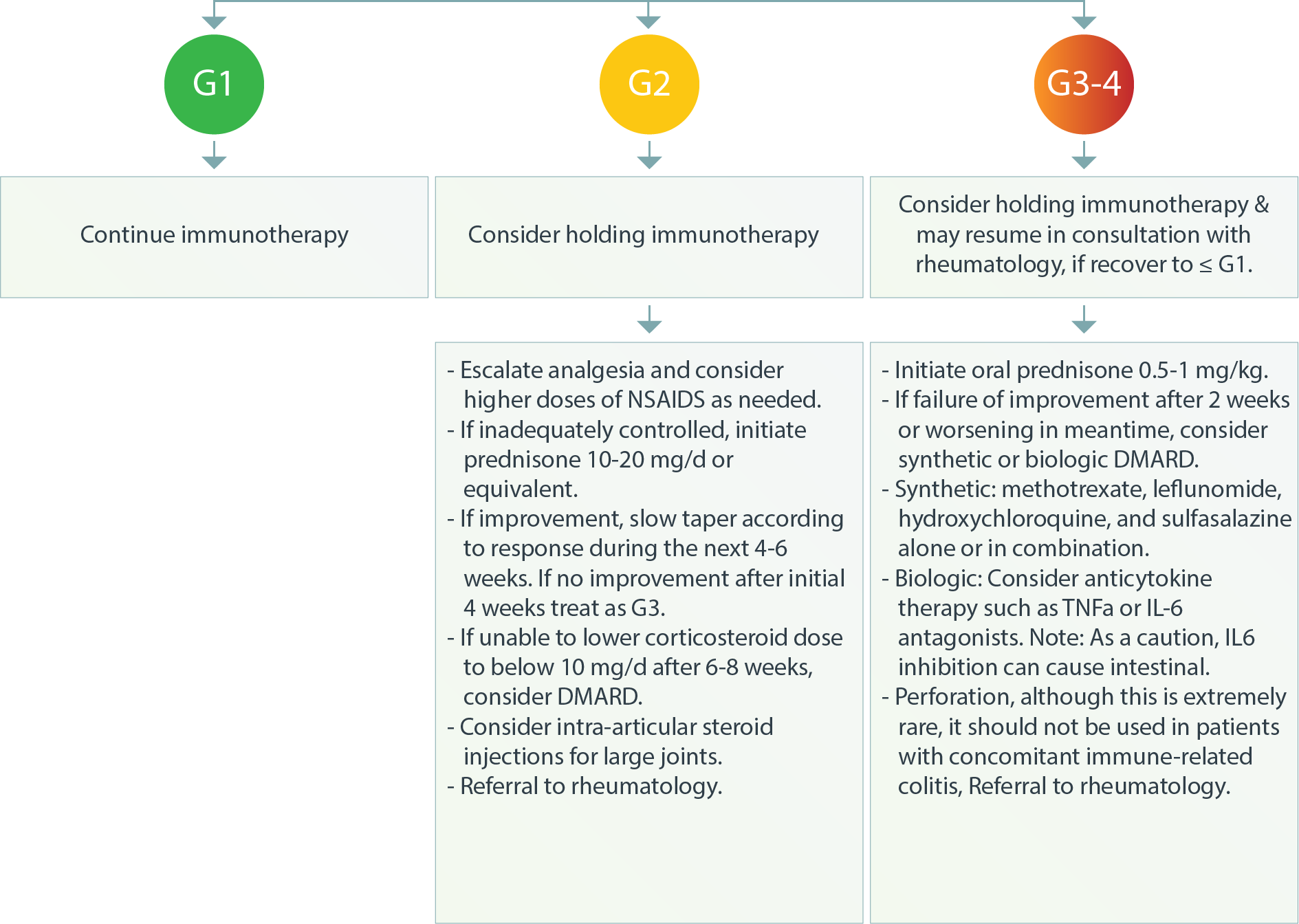
Musculoskeletal – Inflammatory Arthritis3
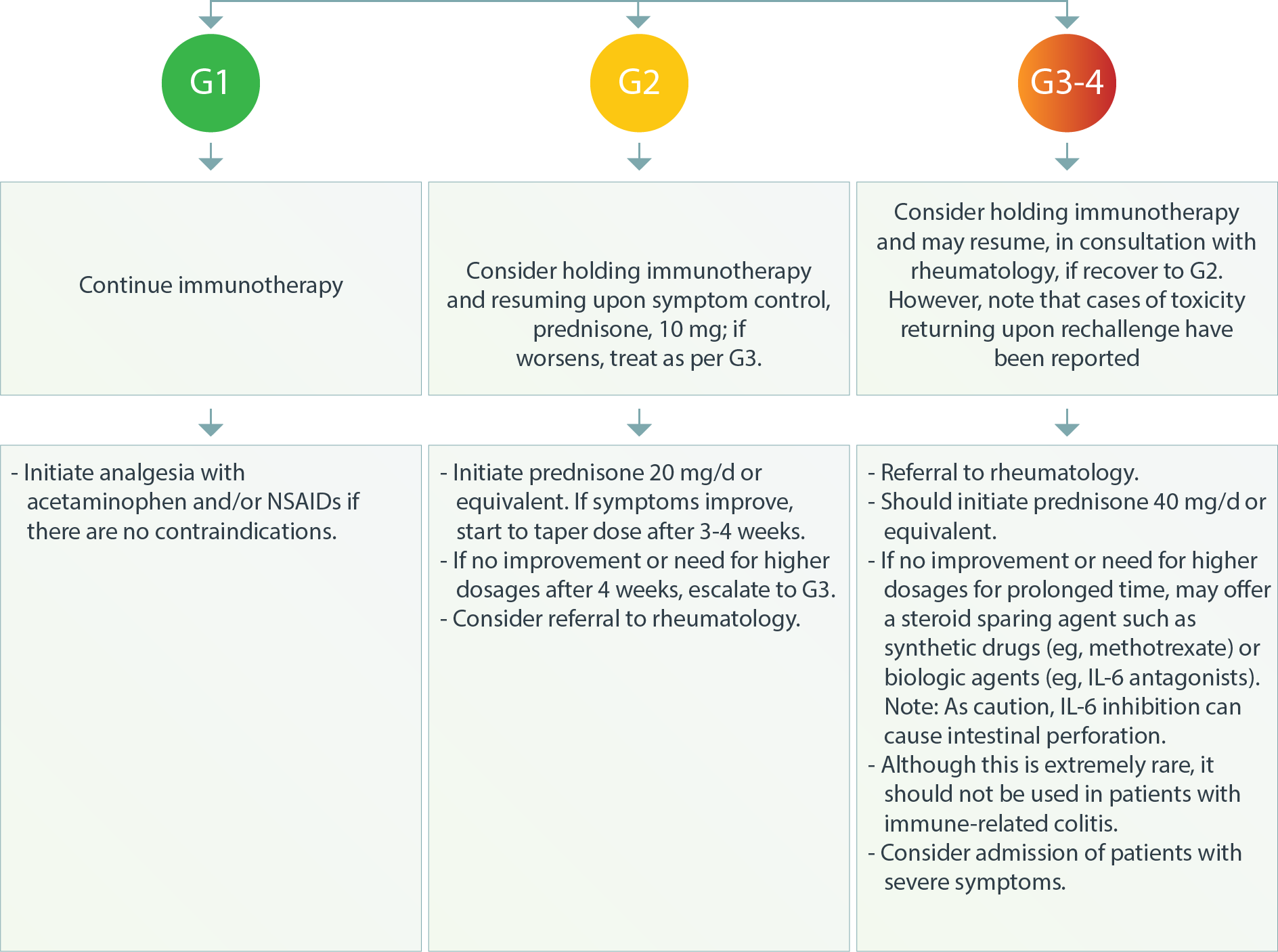
Musculoskeletal–Polymyalgia-LikeSyndrome3
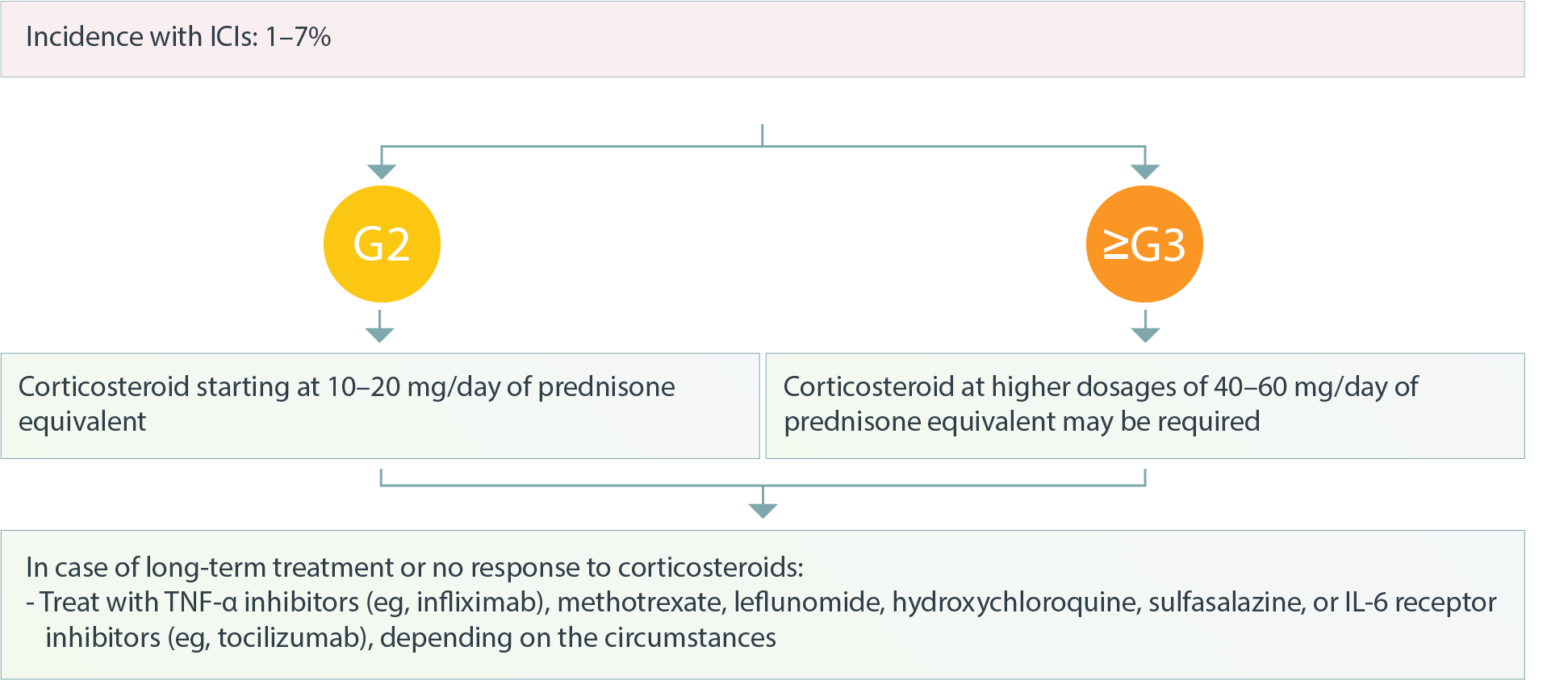
Musculoskeletal Inflammatory Arthritis4
Polymyalgia Rheumatica and Dry Mouth and Sicca Syndrome4

Reference:
- NCCN (National Comprehensive Cancer Network) V.1.2023. Accessed at: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Last accessed: 1-5-2023.
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Accessed at:https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Last accessed: 15-1-2023
- Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. Journal of clinical oncology. 2023;39(36):4073-126
- Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. Journal for immunotherapy of cancer. 2021;9(6)
MSD Egypt, 67 – El Tesseen St., Address Building, Fifth Settlement, New Cairo.
In case you need any updates or you have an inquiry or need to report on an adverse reaction, you can contact:
Veeva Code: EG-KEY-00280
Expiration Date: 07/06/2024





