Pulmonary Toxicity

OVERVIEW
GUIDELINES
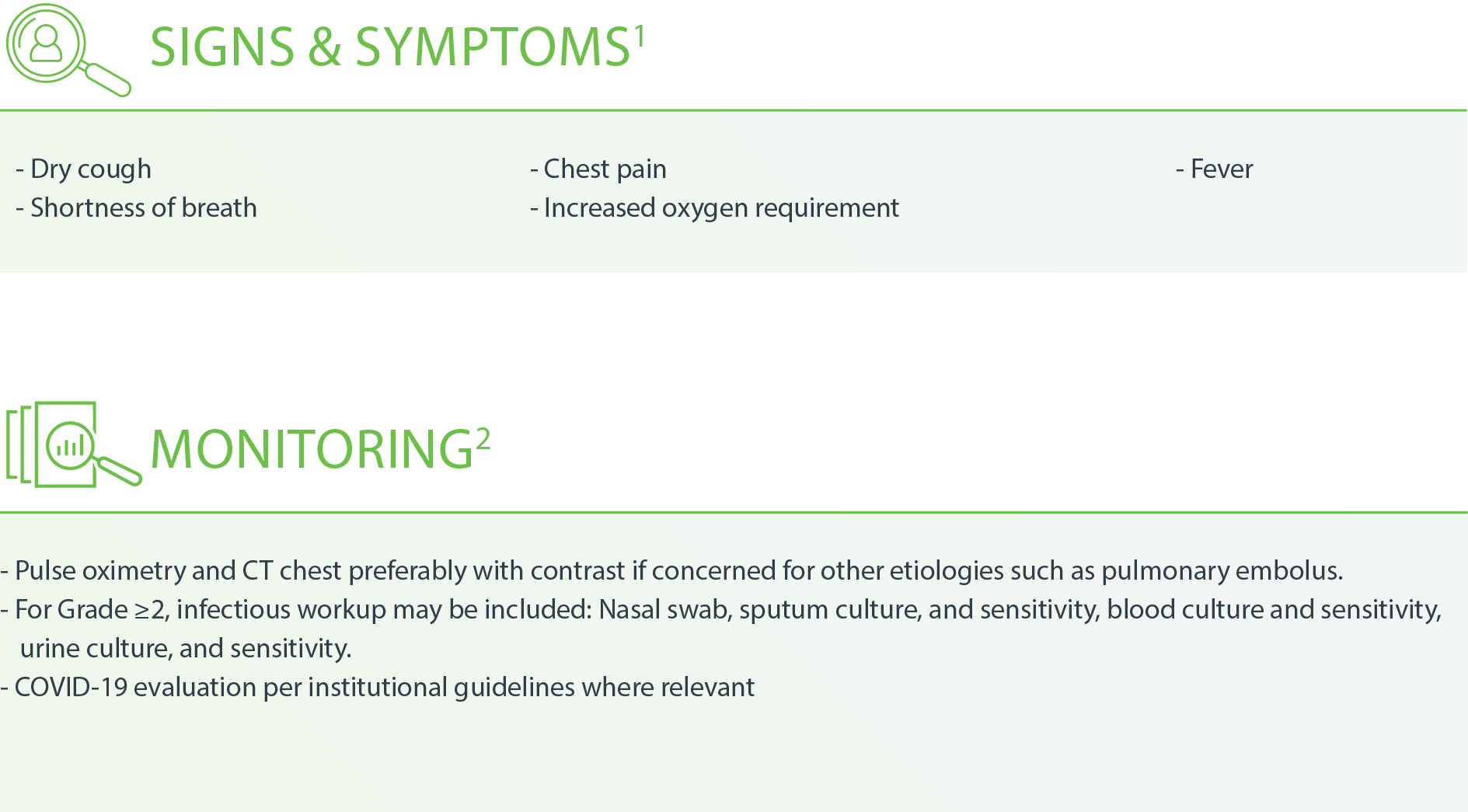
Pulmonary – Pneumonitis1
h CT with contrast to rule out other etiologies if not contraindicated
i Viral pathogen assessment should include COVID-19
j Immunocompromised panel may include CBC with differential, bacterial culture, and Gram stain; AFB culture and stain; fungal immunoassay, culture, and silver stain; CMV, HSV, PJP, and respiratory virus PCR
k Treat until symptoms improve to Grade ≤1, then taper over 4–6 weeks
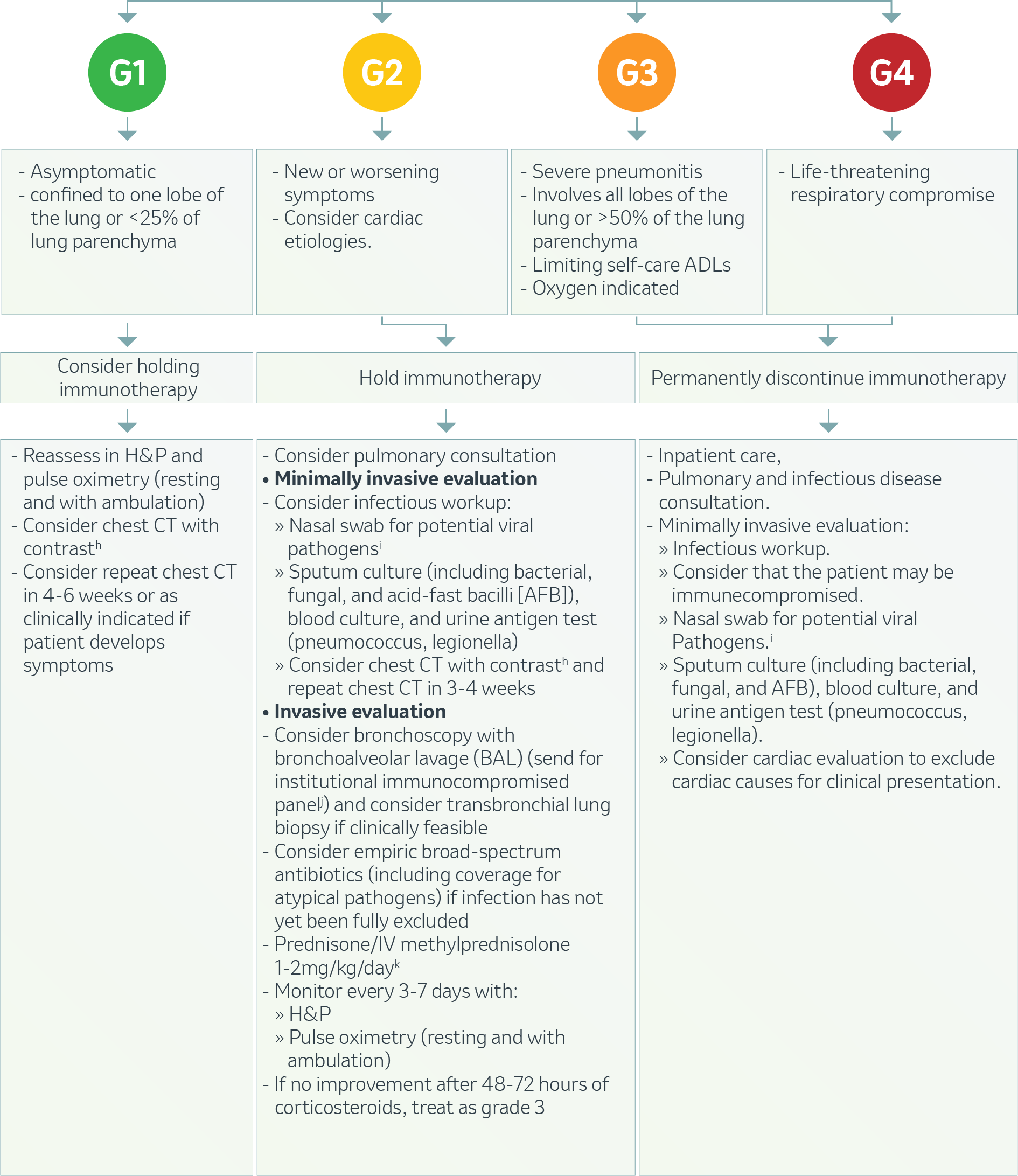
Pulmonary – Pneumonitis2
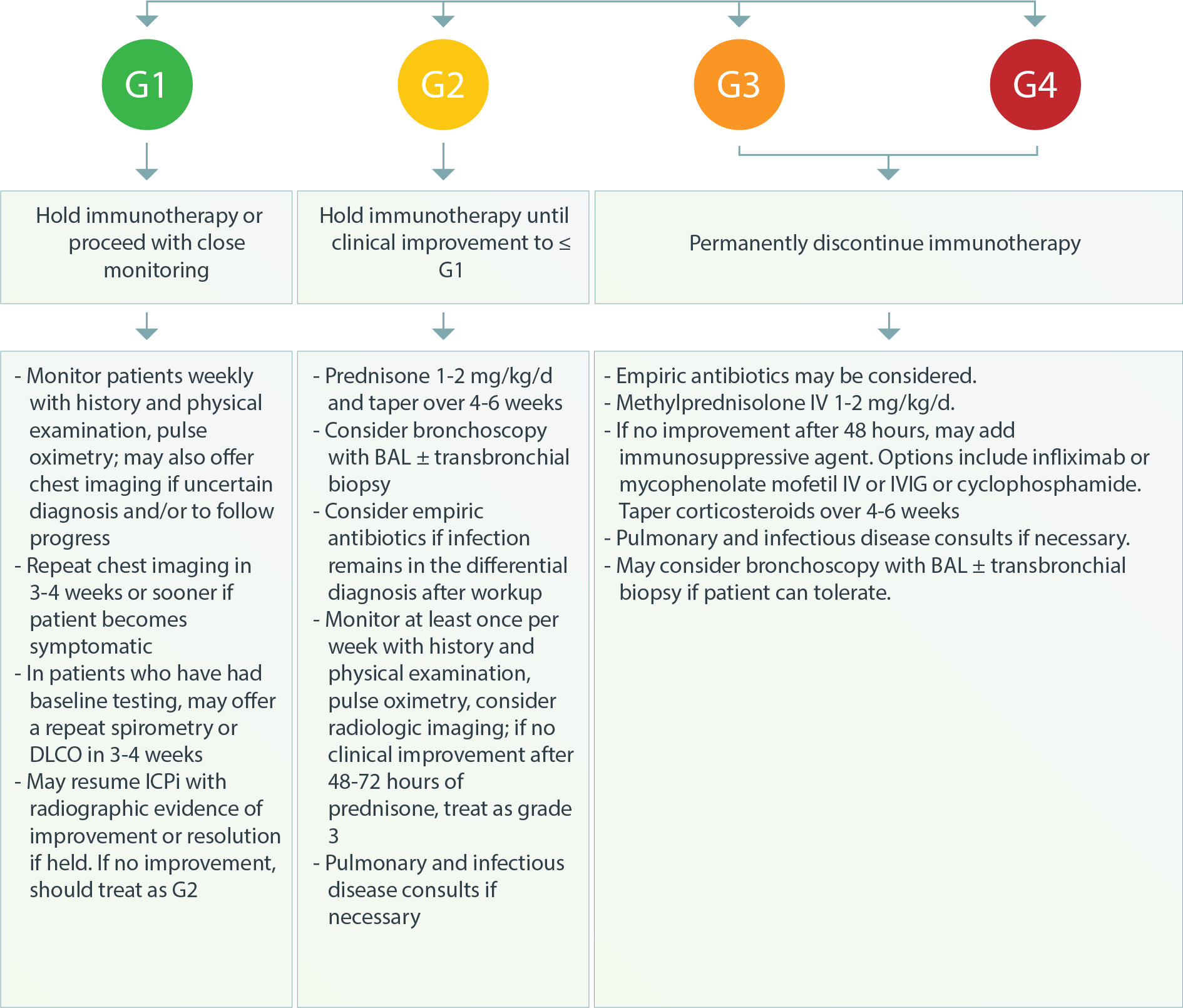
Pulmonary – Pneumonitis3
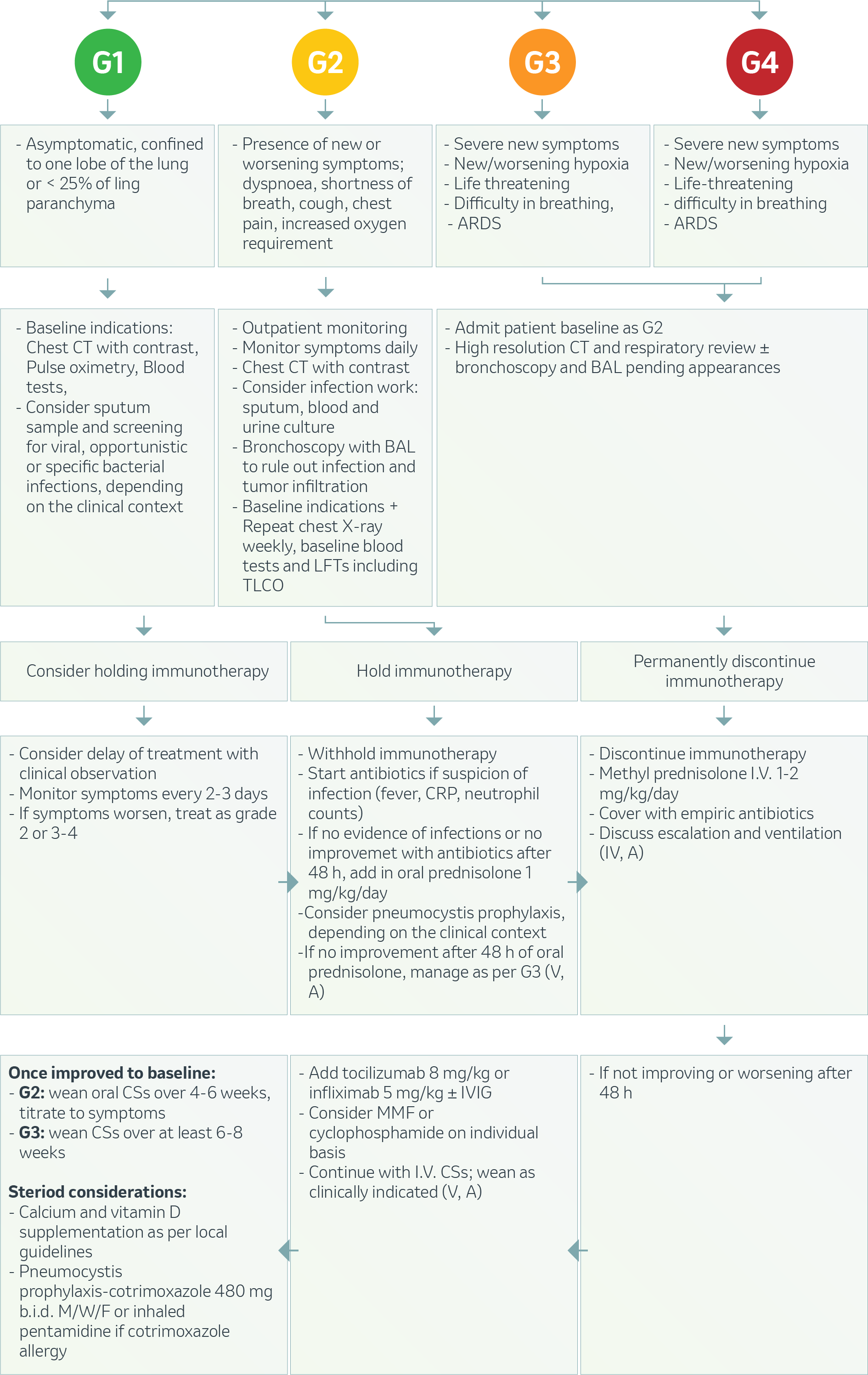
INCIDENCE
Immune-mediated Pneumonitis in Patients Treated with Single-agent Pembrolizumab





ONSET & DURATION
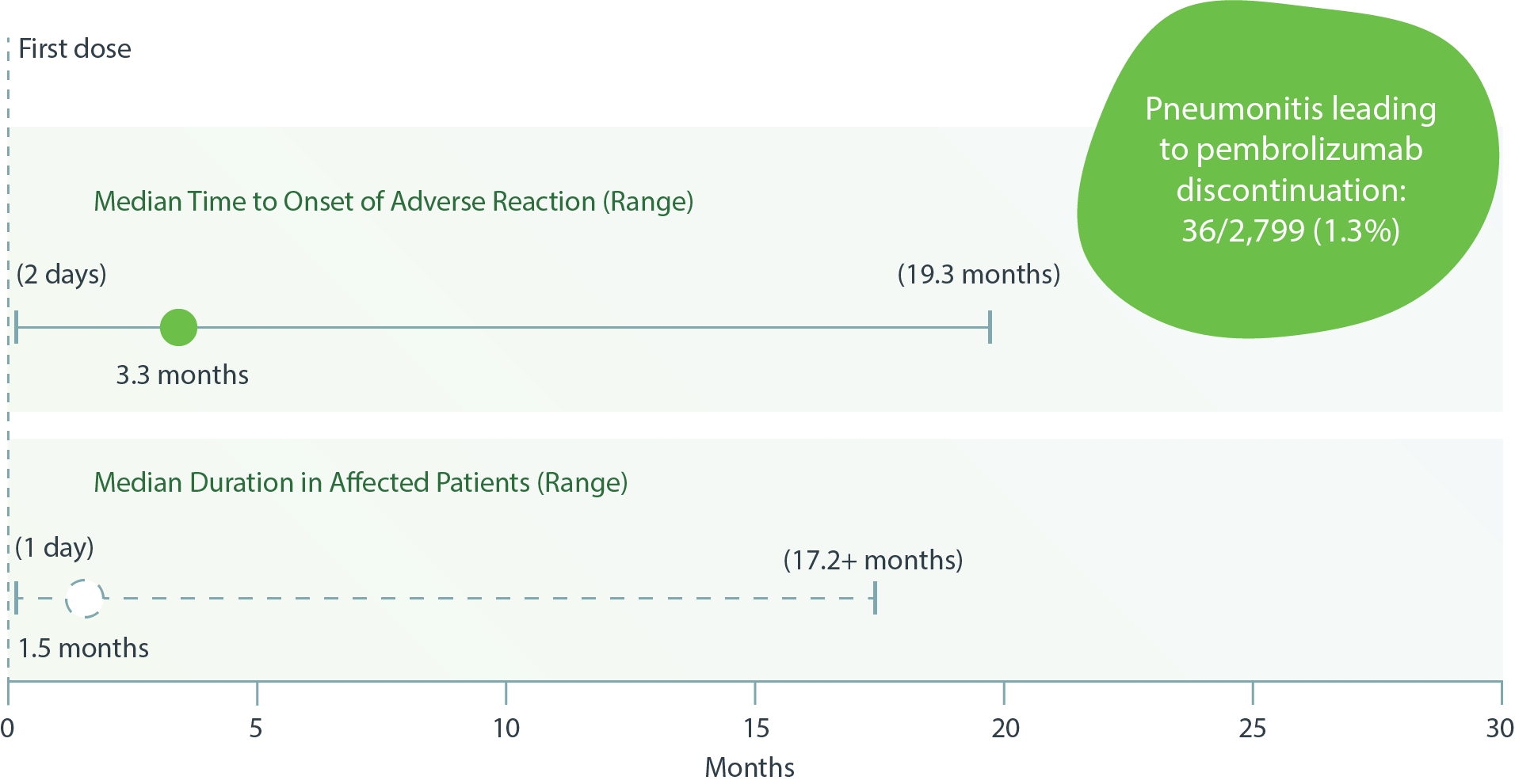
Pembrolizumab: imAE Median Times to Onset and Duration in Affected Patients – Pneumonitis4
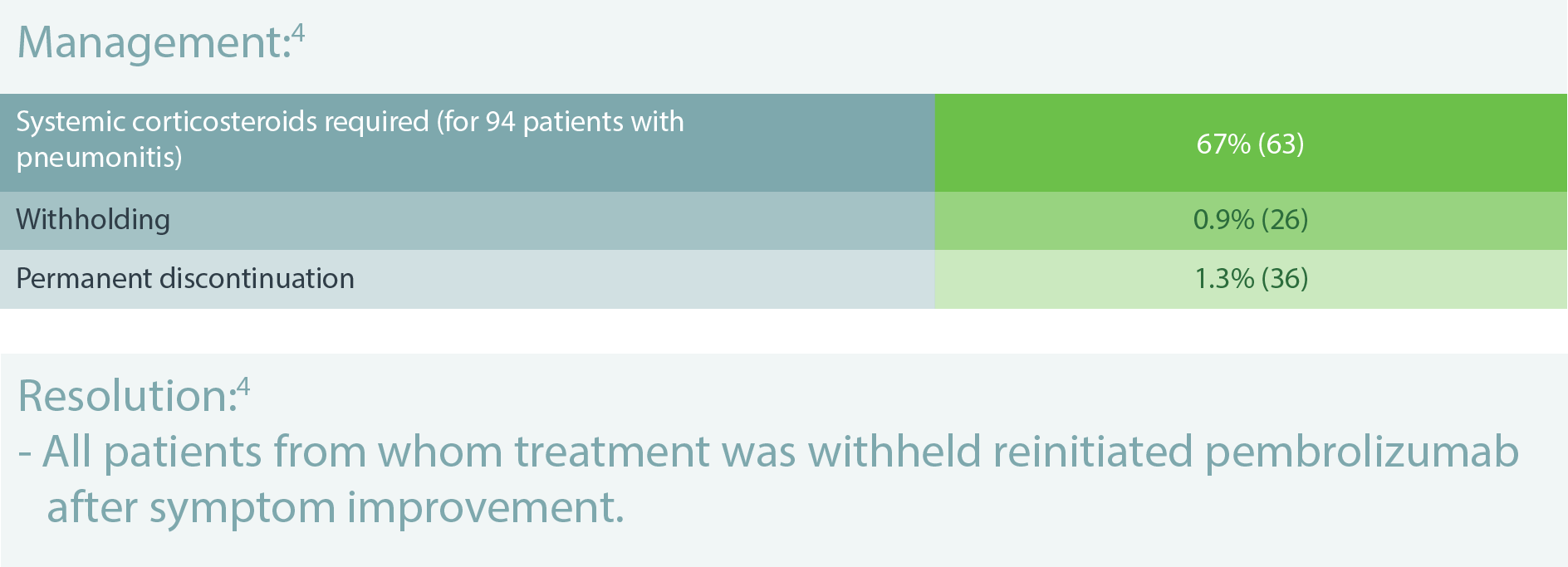
IMMUNE-RELATED PNEUMONITIS IN PATIENTS TREATED WITH PEMBROLIZUMAB (94/2,799)
References:
- NCCN (National Comprehensive Cancer Network) V.1.2023. Accessed at: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Last accessed: 1-5-2023.
- Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. Journal of clinical oncology. 2023;39(36):4073-126
- Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Annals of Oncology. 2022;33(12):1217-38
- Egyptian drug authority KEYTRUDA® leaflet approval date: 23/05/2023
MSD Egypt, 67 – El Tesseen St., Address Building, Fifth Settlement, New Cairo.
In case you need any updates or you have an inquiry or need to report on an adverse reaction, you can contact:
Veeva Code: EG-KEY-00280
Expiration Date: 07/06/2024





